Asthma
| Asthma | |
|---|---|
 | |
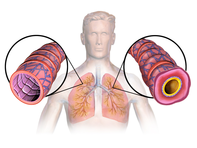
| This is an image of an asthmatics airways, it become swollen and full of mucous. | |
| Pronunciation | |
| Specialty | Pulmonology |
| Symptoms | Recurring episodes of wheezing, coughing, chest tightness, shortness of breath[3] |
| Complications | Gastroesophageal reflux disease (GERD), sinusitis, obstructive sleep apnea |
| Usual onset | Childhood |
| Duration | Long term[4] |
| Causes | Genetic and environmental factors[3] |
| Risk factors | Air pollution, allergens[4] |
| Diagnostic method | Based on symptoms, response to therapy, spirometry[5] |
| Treatment | Avoiding triggers, inhaled corticosteroids, salbutamol[6][7] |
| Frequency | Approx. 262 million (2019)[8] |
| Deaths | Approx. 461,000 (2019)[8] |
Asthma is a common long-term inflammatory disease of the airways of the lungs.[4] Asthma occurs when allergens, pollen, dust, or other particles, are inhaled into the lungs, causing the bronchioles to constrict and produce mucus, which then restricts oxygen flow to the alveoli. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms.[9][10] Symptoms include episodes of wheezing, coughing, chest tightness, and shortness of breath.[3] These may occur a few times a day or a few times per week.[4] Depending on the person, asthma symptoms may become worse at night or with exercise.[4]
Asthma is thought to be caused by a combination of genetic and environmental factors.[3] Environmental factors include exposure to air pollution and allergens.[4] Other potential triggers include medications such as aspirin and beta blockers.[4] Diagnosis is usually based on the pattern of symptoms, response to therapy over time, and spirometry lung function testing.[5] Asthma is classified according to the frequency of symptoms of forced expiratory volume in one second (FEV1), and peak expiratory flow rate.[11] It may also be classified as atopic or non-atopic, where atopy refers to a predisposition toward developing a type 1 hypersensitivity reaction.[12][13]
There is no known cure for asthma, but it can be controlled.[4] Symptoms can be prevented by avoiding triggers, such as allergens and respiratory irritants, and suppressed with the use of inhaled corticosteroids.[6][14] Long-acting beta agonists (LABA) or antileukotriene agents may be used in addition to inhaled corticosteroids if asthma symptoms remain uncontrolled.[15][16] Treatment of rapidly worsening symptoms is usually with an inhaled short-acting beta2 agonist such as salbutamol and corticosteroids taken by mouth.[7] In very severe cases, intravenous corticosteroids, magnesium sulfate, and hospitalization may be required.[17]
In 2019 asthma affected approximately 262 million people and caused approximately 461,000 deaths.[8] Most of the deaths occurred in the developing world.[4] Asthma often begins in childhood,[4] and the rates have increased significantly since the 1960s.[18] Asthma was recognized as early as Ancient Egypt.[19] The word asthma is from the Greek ἆσθμα, âsthma, which means 'panting'.[20]
Signs and symptoms
Asthma is characterized by recurrent episodes of wheezing, shortness of breath, chest tightness, and coughing.[21] Sputum may be produced from the lung by coughing but is often hard to bring up.[22] During recovery from an asthma attack (exacerbation), the sputum may appear pus-like due to high levels of white blood cells called eosinophils.[23] Symptoms are usually worse at night and in the early morning or in response to exercise or cold air.[24] Some people with asthma rarely experience symptoms, usually in response to triggers, whereas others may react frequently and readily and experience persistent symptoms.[25]
Associated conditions
A number of other health conditions occur more frequently in people with asthma, including gastroesophageal reflux disease (GERD), rhinosinusitis, and obstructive sleep apnea.[26] Psychological disorders are also more common,[27] with anxiety disorders occurring in between 16 and 52% and mood disorders in 14–41%.[28] It is not known whether asthma causes psychological problems or psychological problems lead to asthma.[29] Current asthma, but not former asthma, is associated with increased all-cause mortality, heart disease mortality, and chronic lower respiratory tract disease mortality.[30] Asthma, particularly severe asthma, is strongly associated with development of chronic obstructive pulmonary disease (COPD).[31][32][33] Those with asthma, especially if it is poorly controlled, are at increased risk for radiocontrast reactions.[34]
Cavities occur more often in people with asthma.[35] This may be related to the effect of beta2-adrenergic agonists decreasing saliva.[36] These medications may also increase the risk of dental erosions.[36]
Causes
Asthma is caused by a combination of complex and incompletely understood environmental and genetic interactions.[37][38] These influence both its severity and its responsiveness to treatment.[39] It is believed that the recent increased rates of asthma are due to changing epigenetics (heritable factors other than those related to the DNA sequence) and a changing living environment.[40] Asthma that starts before the age of 12 years old is more likely due to genetic influence, while onset after age 12 is more likely due to environmental influence.[41]
Environmental
Many environmental factors have been associated with asthma's development and exacerbation, including allergens, air pollution, and other environmental chemicals.[42] There are some substances that are known to cause asthma in exposed people and they are called asthmagens. Some common asthmagens include ammonia, latex, pesticides, solder and welding fumes, metal or wood dusts, spraying of isocyanate paint in vehicle repair, formaldehyde, glutaraldehyde, anhydrides, glues, dyes, metal working fluids, oil mists, moulds.[43][44] Smoking during pregnancy and after delivery is associated with a greater risk of asthma-like symptoms.[45] Low air quality from environmental factors such as traffic pollution or high ozone levels[46] has been associated with both asthma development and increased asthma severity.[47] Over half of cases in children in the United States occur in areas when air quality is below the EPA standards.[48] Low air quality is more common in low-income and minority communities.[49]
Exposure to indoor volatile organic compounds may be a trigger for asthma; formaldehyde exposure, for example, has a positive association.[50] Phthalates in certain types of PVC are associated with asthma in both children and adults.[51][52] While exposure to pesticides is linked to the development of asthma, a cause and effect relationship has yet to be established.[53][54] A meta-analysis concluded gas stoves are a major risk factor for asthma, finding around one in eight cases in the U.S. could be attributed to these.[55]
The majority of the evidence does not support a causal role between paracetamol (acetaminophen) or antibiotic use and asthma.[56][57] A 2014 systematic review found that the association between paracetamol use and asthma disappeared when respiratory infections were taken into account.[58] Maternal psychological stress during pregnancy is a risk factor for the child to develop asthma.[59]
Asthma is associated with exposure to indoor allergens.[60] Common indoor allergens include dust mites, cockroaches, animal dander (fragments of fur or feathers), and mould.[61][62] Efforts to decrease dust mites have been found to be ineffective on symptoms in sensitized subjects.[63][64] Weak evidence suggests that efforts to decrease mould by repairing buildings may help improve asthma symptoms in adults.[65] Certain viral respiratory infections, such as respiratory syncytial virus and rhinovirus,[20] may increase the risk of developing asthma when acquired as young children.[66] Certain other infections, however, may decrease the risk.[20]
Hygiene hypothesis
The hygiene hypothesis attempts to explain the increased rates of asthma worldwide as a direct and unintended result of reduced exposure, during childhood, to non-pathogenic bacteria and viruses.[67][68] It has been proposed that the reduced exposure to bacteria and viruses is due, in part, to increased cleanliness and decreased family size in modern societies.[69] Exposure to bacterial endotoxin in early childhood may prevent the development of asthma, but exposure at an older age may provoke bronchoconstriction.[70] Evidence supporting the hygiene hypothesis includes lower rates of asthma on farms and in households with pets.[69]
Use of antibiotics in early life has been linked to the development of asthma.[71] Also, delivery via caesarean section is associated with an increased risk (estimated at 20–80%) of asthma – this increased risk is attributed to the lack of healthy bacterial colonization that the newborn would have acquired from passage through the birth canal.[72][73] There is a link between asthma and the degree of affluence which may be related to the hygiene hypothesis as less affluent individuals often have more exposure to bacteria and viruses.[74]
Genetic
| Endotoxin levels | CC genotype | TT genotype |
|---|---|---|
| High exposure | Low risk | High risk |
| Low exposure | High risk | Low risk |
Family history is a risk factor for asthma, with many different genes being implicated.[76] If one identical twin is affected, the probability of the other having the disease is approximately 25%.[76] By the end of 2005, 25 genes had been associated with asthma in six or more separate populations, including GSTM1, IL10, CTLA-4, SPINK5, LTC4S, IL4R and ADAM33, among others.[77] Many of these genes are related to the immune system or modulating inflammation. Even among this list of genes supported by highly replicated studies, results have not been consistent among all populations tested.[77] In 2006 over 100 genes were associated with asthma in one genetic association study alone;[77] more continue to be found.[78]
Some genetic variants may only cause asthma when they are combined with specific environmental exposures.[37] An example is a specific single nucleotide polymorphism in the CD14 region and exposure to endotoxin (a bacterial product). Endotoxin exposure can come from several environmental sources including tobacco smoke, dogs, and farms. Risk for asthma, then, is determined by both a person's genetics and the level of endotoxin exposure.[75]
Medical conditions
A triad of atopic eczema, allergic rhinitis and asthma is called atopy.[79] The strongest risk factor for developing asthma is a history of atopic disease;[66] with asthma occurring at a much greater rate in those who have either eczema or hay fever.[80] Asthma has been associated with eosinophilic granulomatosis with polyangiitis (formerly known as Churg–Strauss syndrome), an autoimmune disease and vasculitis.[81] Individuals with certain types of urticaria may also experience symptoms of asthma.[79]
There is a correlation between obesity and the risk of asthma with both having increased in recent years.[82][83] Several factors may be at play including decreased respiratory function due to a buildup of fat and the fact that adipose tissue leads to a pro-inflammatory state.[84]
Beta blocker medications such as propranolol can trigger asthma in those who are susceptible.[85] Cardioselective beta-blockers, however, appear safe in those with mild or moderate disease.[86][87] Other medications that can cause problems in asthmatics are angiotensin-converting enzyme inhibitors, aspirin, and NSAIDs.[88] Use of acid-suppressing medication (proton pump inhibitors and H2 blockers) during pregnancy is associated with an increased risk of asthma in the child.[89]
Exacerbation
Some individuals will have stable asthma for weeks or months and then suddenly develop an episode of acute asthma. Different individuals react to various factors in different ways.[90] Most individuals can develop severe exacerbation from a number of triggering agents.[90]
Home factors that can lead to exacerbation of asthma include dust, animal dander (especially cat and dog hair), cockroach allergens and mold.[90][91] Perfumes are a common cause of acute attacks in women and children. Both viral and bacterial infections of the upper respiratory tract can worsen the disease.[90] Psychological stress may worsen symptoms – it is thought that stress alters the immune system and thus increases the airway inflammatory response to allergens and irritants.[47][92]
Asthma exacerbations in school-aged children peak in autumn, shortly after children return to school. This might reflect a combination of factors, including poor treatment adherence, increased allergen and viral exposure, and altered immune tolerance. There is limited evidence to guide possible approaches to reducing autumn exacerbations, but while costly, seasonal omalizumab treatment from four to six weeks before school return may reduce autumn asthma exacerbations.[93]
Pathophysiology
Asthma is the result of chronic inflammation of the conducting zone of the airways (most especially the bronchi and bronchioles), which subsequently results in increased contractability of the surrounding smooth muscles. This among other factors leads to bouts of narrowing of the airway and the classic symptoms of wheezing. The narrowing is typically reversible with or without treatment. Occasionally the airways themselves change.[21] Typical changes in the airways include an increase in eosinophils and thickening of the lamina reticularis. Chronically the airways' smooth muscle may increase in size along with an increase in the numbers of mucous glands. Other cell types involved include T lymphocytes, macrophages, and neutrophils. There may also be involvement of other components of the immune system, including cytokines, chemokines, histamine, and leukotrienes among others.[20]
Diagnosis
While asthma is a well-recognized condition, there is not one universal agreed-upon definition.[20] It is defined by the Global Initiative for Asthma as "a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role. The chronic inflammation is associated with airway hyper-responsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness and coughing particularly at night or in the early morning. These episodes are usually associated with widespread but variable airflow obstruction within the lung that is often reversible either spontaneously or with treatment".[21]
There is currently no precise test for the diagnosis, which is typically based on the pattern of symptoms and response to therapy over time.[5][20] Asthma may be suspected if there is a history of recurrent wheezing, coughing or difficulty breathing and these symptoms occur or worsen due to exercise, viral infections, allergens or air pollution.[94] Spirometry is then used to confirm the diagnosis.[94] In children under the age of six the diagnosis is more difficult as they are too young for spirometry.[95]
Spirometry
Spirometry is recommended to aid in diagnosis and management.[96][97] It is the single best test for asthma. If the FEV1 measured by this technique improves more than 12% and increases by at least 200 millilitres following administration of a bronchodilator such as salbutamol, this is supportive of the diagnosis. It however may be normal in those with a history of mild asthma, not currently acting up.[20] As caffeine is a bronchodilator in people with asthma, the use of caffeine before a lung function test may interfere with the results.[98] Single-breath diffusing capacity can help differentiate asthma from COPD.[20] It is reasonable to perform spirometry every one or two years to follow how well a person's asthma is controlled.[99]
Others
The methacholine challenge involves the inhalation of increasing concentrations of a substance that causes airway narrowing in those predisposed. If negative it means that a person does not have asthma; if positive, however, it is not specific for the disease.[20]
Other supportive evidence includes: a ≥20% difference in peak expiratory flow rate on at least three days in a week for at least two weeks, a ≥20% improvement of peak flow following treatment with either salbutamol, inhaled corticosteroids or prednisone, or a ≥20% decrease in peak flow following exposure to a trigger.[100] Testing peak expiratory flow is more variable than spirometry, however, and thus not recommended for routine diagnosis. It may be useful for daily self-monitoring in those with moderate to severe disease and for checking the effectiveness of new medications. It may also be helpful in guiding treatment in those with acute exacerbations.[101]
Classification
| Severity | Symptom frequency | Night-time symptoms | %FEV1 of predicted | FEV1 variability | SABA use |
|---|---|---|---|---|---|
| Intermittent | ≤2/week | ≤2/month | ≥80% | <20% | ≤2 days/week |
| Mild persistent | >2/week | 3–4/month | ≥80% | 20–30% | >2 days/week |
| Moderate persistent | Daily | >1/week | 60–80% | >30% | daily |
| Severe persistent | Continuously | Frequent (7/week) | <60% | >30% | ≥twice/day |
Asthma is clinically classified according to the frequency of symptoms, forced expiratory volume in one second (FEV1), and peak expiratory flow rate.[11] Asthma may also be classified as atopic (extrinsic) or non-atopic (intrinsic), based on whether symptoms are precipitated by allergens (atopic) or not (non-atopic).[12] While asthma is classified based on severity, at the moment there is no clear method for classifying different subgroups of asthma beyond this system.[102] Finding ways to identify subgroups that respond well to different types of treatments is a current critical goal of asthma research.[102] Recently, asthma has been classified based on whether it is associated with type 2 or non–type 2 inflammation. This approach to immunologic classification is driven by a developing understanding of the underlying immune processes and by the development of therapeutic approaches that target type 2 inflammation.[103]
Although asthma is a chronic obstructive condition, it is not considered as a part of chronic obstructive pulmonary disease, as this term refers specifically to combinations of disease that are irreversible such as bronchiectasis and emphysema.[104] Unlike these diseases, the airway obstruction in asthma is usually reversible; however, if left untreated, the chronic inflammation from asthma can lead the lungs to become irreversibly obstructed due to airway remodelling.[105] In contrast to emphysema, asthma affects the bronchi, not the alveoli.[106] The combination of asthma with a component of irreversible airway obstruction has been termed the asthma-chronic obstructive disease (COPD) overlap syndrome (ACOS). Compared to other people with "pure" asthma or COPD, people with ACOS exhibit increased morbidity, mortality and possibly more comorbidities.[107]
Asthma exacerbation
| Near-fatal | High PaCO2, or requiring mechanical ventilation, or both | |
|---|---|---|
| Life-threatening (any one of) | ||
| Clinical signs | Measurements | |
| Altered level of consciousness | Peak flow < 33% | |
| Exhaustion | Oxygen saturation < 92% | |
| Arrhythmia | PaO2 < 8 kPa | |
| Low blood pressure | "Normal" PaCO2 | |
| Cyanosis | ||
| Silent chest | ||
| Poor respiratory effort | ||
| Acute severe (any one of) | ||
| Peak flow 33–50% | ||
| Respiratory rate ≥ 25 breaths per minute | ||
| Heart rate ≥ 110 beats per minute | ||
| Unable to complete sentences in one breath | ||
| Moderate | Worsening symptoms | |
| Peak flow 50–80% best or predicted | ||
| No features of acute severe asthma | ||
An acute asthma exacerbation is commonly referred to as an asthma attack. The classic symptoms are shortness of breath, wheezing, and chest tightness.[20] The wheezing is most often when breathing out.[109] While these are the primary symptoms of asthma,[110] some people present primarily with coughing, and in severe cases, air motion may be significantly impaired such that no wheezing is heard.[108] In children, chest pain is often present.[111]
Signs occurring during an asthma attack include the use of accessory muscles of respiration (sternocleidomastoid and scalene muscles of the neck), there may be a paradoxical pulse (a pulse that is weaker during inhalation and stronger during exhalation), and over-inflation of the chest.[112] A blue colour of the skin and nails may occur from lack of oxygen.[113]
In a mild exacerbation the peak expiratory flow rate (PEFR) is ≥200 L/min, or ≥50% of the predicted best.[114] Moderate is defined as between 80 and 200 L/min, or 25% and 50% of the predicted best, while severe is defined as ≤ 80 L/min, or ≤25% of the predicted best.[114]
Acute severe asthma, previously known as status asthmaticus, is an acute exacerbation of asthma that does not respond to standard treatments of bronchodilators and corticosteroids.[115] Half of cases are due to infections with others caused by allergen, air pollution, or insufficient or inappropriate medication use.[115]
Brittle asthma is a kind of asthma distinguishable by recurrent, severe attacks.[108] Type 1 brittle asthma is a disease with wide peak flow variability, despite intense medication. Type 2 brittle asthma is background well-controlled asthma with sudden severe exacerbations.[108]
Exercise-induced
Exercise can trigger bronchoconstriction both in people with or without asthma.[116] It occurs in most people with asthma and up to 20% of people without asthma.[116] Exercise-induced bronchoconstriction is common in professional athletes. The highest rates are among cyclists (up to 45%), swimmers, and cross-country skiers.[117] While it may occur with any weather conditions, it is more common when it is dry and cold.[118] Inhaled beta2 agonists do not appear to improve athletic performance among those without asthma;[119] however, oral doses may improve endurance and strength.[120][121]
Occupational
Asthma as a result of (or worsened by) workplace exposures is a commonly reported occupational disease.[122] Many cases, however, are not reported or recognized as such.[123][124] It is estimated that 5–25% of asthma cases in adults are work-related. A few hundred different agents have been implicated, with the most common being isocyanates, grain and wood dust, colophony, soldering flux, latex, animals, and aldehydes. The employment associated with the highest risk of problems include those who spray paint, bakers and those who process food, nurses, chemical workers, those who work with animals, welders, hairdressers and timber workers.[122]
Aspirin-exacerbated respiratory disease
Aspirin-exacerbated respiratory disease (AERD), also known as aspirin-induced asthma, affects up to 9% of asthmatics.[125] AERD consists of asthma, nasal polyps, sinus disease, and respiratory reactions to aspirin and other NSAID medications (such as ibuprofen and naproxen).[126] People often also develop loss of smell and most experience respiratory reactions to alcohol.[127]
Alcohol-induced asthma
Alcohol may worsen asthmatic symptoms in up to a third of people.[128] This may be even more common in some ethnic groups such as the Japanese and those with aspirin-exacerbated respiratory disease.[128] Other studies have found improvement in asthmatic symptoms from alcohol.[128]
Non-atopic asthma
Non-atopic asthma, also known as intrinsic or non-allergic, makes up between 10 and 33% of cases. There is negative skin test to common inhalant allergens. Often it starts later in life, and women are more commonly affected than men. Usual treatments may not work as well.[129] The concept that "non-atopic" is synonymous with "non-allergic" is called into question by epidemiological data that the prevalence of asthma is closely related to the serum IgE level standardized for age and sex (P<0.0001), indicating that asthma is almost always associated with some sort of IgE-related reaction and therefore has an allergic basis, although not all the allergic stimuli that cause asthma appear to have been included in the battery of aeroallergens studied (the "missing antigen(s)" hypothesis).[130] For example, an updated systematic review and meta-analysis of population-attributable risk (PAR) of Chlamydia pneumoniae biomarkers in chronic asthma found that the PAR for C. pneumoniae-specific IgE was 47%.[131]
Infectious asthma
Infectious asthma is an easily identified clinical presentation.[132] When queried, asthma patients may report that their first asthma symptoms began after an acute lower respiratory tract illness. This type of history has been labelled the "infectious asthma" (IA) syndrome,[133] or as "asthma associated with infection" (AAWI)[134] to distinguish infection-associated asthma initiation from the well known association of respiratory infections with asthma exacerbations. Reported clinical prevalences of IA for adults range from around 40% in a primary care practice[133] to 70% in a speciality practice treating mainly severe asthma patients.[135] Additional information on the clinical prevalence of IA in adult-onset asthma is unavailable because clinicians are not trained to elicit this type of history routinely, and recollection in child-onset asthma is challenging. A population-based incident case-control study in a geographically defined area of Finland reported that 35.8% of new-onset asthma cases had experienced acute bronchitis or pneumonia in the year preceding asthma onset, representing a significantly higher risk compared to randomly selected controls (odds ratio 7.2, 95% confidence interval 5.2–10).[136]
Phenotyping and endotyping
Asthma phenotyping and endotyping has emerged as a novel approach to asthma classification inspired by precision medicine which separates the clinical presentations of asthma, or asthma phenotypes, from their underlying causes, or asthma endotypes. The best-supported endotypic distinction is the type 2-high/type 2-low distinction. Classification based on type 2 inflammation is useful in predicting which patients will benefit from targeted biologic therapy.[137][138]
Differential diagnosis
Many other conditions can cause symptoms similar to those of asthma. In children, symptoms may be due to other upper airway diseases such as allergic rhinitis and sinusitis, as well as other causes of airway obstruction including foreign body aspiration, tracheal stenosis, laryngotracheomalacia, vascular rings, enlarged lymph nodes or neck masses.[139] Bronchiolitis and other viral infections may also produce wheezing.[140] According to European Respiratory Society, it may not be suitable to label wheezing preschool children with the term asthma because there is lack of clinical data on inflammation in airways.[141] In adults, COPD, congestive heart failure, airway masses, as well as drug-induced coughing due to ACE inhibitors may cause similar symptoms. In both populations vocal cord dysfunction may present similarly.[139]
Chronic obstructive pulmonary disease can coexist with asthma and can occur as a complication of chronic asthma. After the age of 65, most people with obstructive airway disease will have asthma and COPD. In this setting, COPD can be differentiated by increased airway neutrophils, abnormally increased wall thickness, and increased smooth muscle in the bronchi. However, this level of investigation is not performed due to COPD and asthma sharing similar principles of management: corticosteroids, long-acting beta-agonists, and smoking cessation.[142] It closely resembles asthma in symptoms, is correlated with more exposure to cigarette smoke, an older age, less symptom reversibility after bronchodilator administration, and decreased likelihood of family history of atopy.[143][144]
Prevention
The evidence for the effectiveness of measures to prevent the development of asthma is weak.[145] The World Health Organization recommends decreasing risk factors such as tobacco smoke, air pollution, chemical irritants including perfume, and the number of lower respiratory infections.[146][147] Other efforts that show promise include: limiting smoke exposure in utero, breastfeeding, and increased exposure to daycare or large families, but none are well supported enough to be recommended for this indication.[145]
Early pet exposure may be useful.[148] Results from exposure to pets at other times are inconclusive[149] and it is only recommended that pets be removed from the home if a person has allergic symptoms to said pet.[150]
Dietary restrictions during pregnancy or when breastfeeding have not been found to be effective at preventing asthma in children and are not recommended.[150] Omega-3 consumption, Mediterranean diet and antioxidants have been suggested by some studies to potentially help prevent crises but the evidence is still inconclusive.[151]
Reducing or eliminating compounds known to sensitive people from the workplace may be effective.[122] It is not clear if annual influenza vaccinations affect the risk of exacerbations.[152] Immunization, however, is recommended by the World Health Organization.[153] Smoking bans are effective in decreasing exacerbations of asthma.[154]
Management
While there is no cure for asthma, symptoms can typically be improved.[155] The most effective treatment for asthma is identifying triggers, such as cigarette smoke, pets or other allergens, and eliminating exposure to them. If trigger avoidance is insufficient, the use of medication is recommended. Pharmaceutical drugs are selected based on, among other things, the severity of illness and the frequency of symptoms. Specific medications for asthma are broadly classified into fast-acting and long-acting categories.[156][157] The medications listed below have demonstrated efficacy in improving asthma symptoms; however, real world use-effectiveness is limited as around half of people with asthma worldwide remain sub-optimally controlled, even when treated.[158][159][160] People with asthma may remain sub-optimally controlled either because optimum doses of asthma medications do not work (called "refractory" asthma) or because individuals are either unable (e.g. inability to afford treatment, poor inhaler technique) or unwilling (e.g., wish to avoid side effects of corticosteroids) to take optimum doses of prescribed asthma medications (called "difficult to treat" asthma). In practice, it is not possible to distinguish "refractory" from "difficult to treat" categories for patients who have never taken optimum doses of asthma medications. A related issue is that the asthma efficacy trials upon which the pharmacological treatment guidelines are based have systematically excluded the majority of people with asthma.[161][162] For example, asthma efficacy treatment trials always exclude otherwise eligible people who smoke, and smoking diminishes the efficacy of inhaled corticosteroids, the mainstay of asthma control management.[163][164][165]
Bronchodilators are recommended for short-term relief of symptoms. In those with occasional attacks, no other medication is needed. If mild persistent disease is present (more than two attacks a week), low-dose inhaled corticosteroids or alternatively, a leukotriene antagonist or a mast cell stabilizer by mouth is recommended. For those who have daily attacks, a higher dose of inhaled corticosteroids is used. In a moderate or severe exacerbation, corticosteroids by mouth are added to these treatments.[7]
People with asthma have higher rates of anxiety, psychological stress, and depression.[166][167] This is associated with poorer asthma control.[166] Cognitive behavioural therapy may improve quality of life, asthma control, and anxiety levels in people with asthma.[166]
Improving people's knowledge about asthma and using a written action plan has been identified as an important component of managing asthma.[168] Providing educational sessions that include information specific to a person's culture is likely effective.[169] More research is necessary to determine if increasing preparedness and knowledge of asthma among school staff and families using home-based and school interventions results in long term improvements in safety for children with asthma.[170][171][172] School-based asthma self-management interventions, which attempt to improve knowledge of asthma, its triggers and the importance of regular practitioner review, may reduce hospital admissions and emergency department visits. These interventions may also reduce the number of days children experience asthma symptoms and may lead to small improvements in asthma-related quality of life.[173] More research is necessary to determine if shared decision-making is helpful for managing adults with asthma[174] or if a personalized asthma action plan is effective and necessary.[175] Some people with asthma use pulse oximeters to monitor their own blood oxygen levels during an asthma attack. However, there is no evidence regarding the use in these instances.[176]
Lifestyle modification
Avoidance of triggers is a key component of improving control and preventing attacks. The most common triggers include allergens, smoke (from tobacco or other sources), air pollution, nonselective beta-blockers, and sulfite-containing foods.[177][178] Cigarette smoking and second-hand smoke (passive smoke) may reduce the effectiveness of medications such as corticosteroids.[179] Laws that limit smoking decrease the number of people hospitalized for asthma.[154] Dust mite control measures, including air filtration, chemicals to kill mites, vacuuming, mattress covers and other methods had no effect on asthma symptoms.[63] There is insufficient evidence to suggest that dehumidifiers are helpful for controlling asthma.[180]
Overall, exercise is beneficial in people with stable asthma.[181] Yoga could provide small improvements in quality of life and symptoms in people with asthma.[182] More research is necessary to determine how effective weight loss is in improving quality of life, the usage of health care services, and adverse effects for people of all ages with asthma.[183][184]
Findings suggest that the Wim Hof Method may reduce inflammation in healthy and non-healthy participants as it increases epinephrine levels, causing an increase in interleukin-10 and a decrease in pro-inflammatory cytokines.[185]
Medications
Medications used to treat asthma are divided into two general classes: quick-relief medications used to treat acute symptoms; and long-term control medications used to prevent further exacerbation.[156] Antibiotics are generally not needed for sudden worsening of symptoms or for treating asthma at any time.[186][187]
Medications for asthma exacerbations

- Short-acting beta2-adrenoceptor agonists (SABAs), such as salbutamol (albuterol USAN) are the first-line treatment for asthma symptoms.[7] They are recommended before exercise in those with exercise-induced symptoms.[188]
- Anticholinergic medications, such as ipratropium, provide additional benefit when used in combination with SABA in those with moderate or severe symptoms and may prevent hospitalizations.[7][189][190] Anticholinergic bronchodilators can also be used if a person cannot tolerate a SABA.[104] If a child requires admission to hospital additional ipratropium does not appear to help over a SABA.[191] For children over 2 years old with acute asthma symptoms, inhaled anticholinergic medications taken alone is safe but is not as effective as inhaled SABA or SABA combined with inhaled anticholinergic medication.[192][189] Adults who receive combined inhaled medications, which include short-acting anticholinergics and SABA, may be at risk for increased adverse effects such as experiencing a tremor, agitation, and heart beat palpitations compared to people who are treated with SABAs alone.[190]
- Older, less selective adrenergic agonists, such as inhaled epinephrine, have similar efficacy to SABAs.[193] They are, however, not recommended due to concerns regarding excessive cardiac stimulation.[194]
- Corticosteroids can also help with the acute phase of an exacerbation because of their antiinflamatory properties. The benefit of systemic and oral corticosteroids is well established. Inhaled or nebulized corticosteroids can also be used.[151] For adults and children who are in the hospital due to acute asthma, systemic (IV) corticosteroids improve symptoms.[195][196] A short course of corticosteroids after an acute asthma exacerbation may help prevent relapses and reduce hospitalizations.[197]
- Other remedies, less established, are intravenous or nebulized magnesium sulfate and helium mixed with oxygen. Aminophylline could be used with caution as well.[151]
- Mechanical ventilation is the last resort in case of severe hypoxemia.[151]
- Intravenous administration of the drug aminophylline does not provide an improvement in bronchodilation when compared to standard inhaled beta2 agonist treatment.[198] Aminophylline treatment is associated with more adverse effects compared to inhaled beta2 agonist treatment.[198]
Long–term control

- Corticosteroids are generally considered the most effective treatment available for long-term control.[156] Inhaled forms are usually used except in the case of severe persistent disease, in which oral corticosteroids may be needed.[156] Dosage depends on the severity of symptoms.[199] High dosage and long-term use might lead to the appearance of common adverse effects which are growth delay, adrenal suppression, and osteoporosis.[151] Continuous (daily) use of an inhaled corticosteroid, rather than its intermitted use, seems to provide better results in controlling asthma exacerbations.[151] Commonly used corticosteroids are budesonide, fluticasone, mometasone and ciclesonide.[151]
- Long-acting beta-adrenoceptor agonists (LABA) such as salmeterol and formoterol can improve asthma control, at least in adults, when given in combination with inhaled corticosteroids.[200][201] In children this benefit is uncertain.[200][202][201] When used without steroids they increase the risk of severe side-effects,[203] and with corticosteroids they may slightly increase the risk.[204][205] Evidence suggests that for children who have persistent asthma, a treatment regime that includes LABA added to inhaled corticosteroids may improve lung function but does not reduce the amount of serious exacerbations.[206] Children who require LABA as part of their asthma treatment may need to go to the hospital more frequently.[206]
- Leukotriene receptor antagonists (anti-leukotriene agents such as montelukast and zafirlukast) may be used in addition to inhaled corticosteroids, typically also in conjunction with a LABA.[16][207][208][209] For adults or adolescents who have persistent asthma that is not controlled very well, the addition of anti-leukotriene agents along with daily inhaled corticosteriods improves lung function and reduces the risk of moderate and severe asthma exacerbations.[208] Anti-leukotriene agents may be effective alone for adolescents and adults; however, there is no clear research suggesting which people with asthma would benefit from anti-leukotriene receptor alone.[210] In those under five years of age, anti-leukotriene agents were the preferred add-on therapy after inhaled corticosteroids.[151][211] A 2013 Cochrane systematic review concluded that anti-leukotriene agents appear to be of little benefit when added to inhaled steroids for treating children.[212] A similar class of drugs, 5-LOX inhibitors, may be used as an alternative in the chronic treatment of mild to moderate asthma among older children and adults.[16][213] As of 2013[update] there is one medication in this family known as zileuton.[16]
- Mast cell stabilizers (such as cromolyn sodium) are safe alternatives to corticosteroids but not preferred because they have to be administered frequently.[156][16]
- Oral theophyllines are sometimes used for controlling chronic asthma, but their used is minimized due to side effects.[151]
- Omalizumab, a monoclonal antibody against IgE, is a novel way to lessen exacerbations by decreasing the levels of circulating IgE that play a significant role at allergic asthma.[151][214]
- Anticholinergic medications such as ipratropium bromide have not been shown to be beneficial for treating chronic asthma in children over 2 years old,[215] and are not suggested for routine treatment of chronic asthma in adults.[216]
- There is no strong evidence to recommend chloroquine medication as a replacement for taking corticosteroids by mouth (for those who are not able to tolerate inhaled steroids).[217] Methotrexate is not suggested as a replacement for taking corticosteriods by mouth ("steroid-sparing") due to the adverse effects associated with taking methotrexate and the minimal relief provided for asthma symptoms.[218]
- Macrolide antibiotics, particularly the azalide macrolide azithromycin, are a recently added Global Initiative for Asthma (GINA)-recommended treatment option for both eosinophilic and non-eosinophilic severe, refractory asthma based on azithromycin's efficacy in reducing moderate and severe exacerbations combined.[219][220] Azithromycin's mechanism of action is not established, and could involve pathogen- and/or host-directed anti-inflammatory activities.[221] Limited clinical observations suggest that some patients with new-onset asthma and with "difficult-to-treat" asthma (including those with the asthma-COPD overlap syndrome – ACOS) may respond dramatically to azithromycin.[222][135] However, these groups of asthma patients have not been studied in randomized treatment trials and patient selection needs to be carefully individualized.
- A 2024 study indicates that commonly used diabetes medications may lower asthma attacks by up to 70%.[223] The research examined metformin and GLP-1 drugs such as Ozempic (semaglutide), Mounjaro (tirzepatide), and Saxenda (liraglutide). Among nearly 13,000 participants with both diabetes and asthma, metformin reduced the risk of asthma attacks by 30%, with an additional 40% reduction when combined with a GLP-1 drug.[224]
For children with asthma which is well-controlled on combination therapy of inhaled corticosteroids (ICS) and long-acting beta2-agonists (LABA), the benefits and harms of stopping LABA and stepping down to ICS-only therapy are uncertain.[225] In adults who have stable asthma while they are taking a combination of LABA and inhaled corticosteroids (ICS), stopping LABA may increase the risk of asthma exacerbations that require treatment with corticosteroids by mouth.[226] Stopping LABA probably makes little or no important difference to asthma control or asthma-related quality of life.[226] Whether or not stopping LABA increases the risk of serious adverse events or exacerbations requiring an emergency department visit or hospitalization is uncertain.[226]
Delivery methods
Medications are typically provided as metered-dose inhalers (MDIs) in combination with an inhaler spacer or as a dry powder inhaler. The spacer is a plastic cylinder that mixes the medication with air, making it easier to receive a full dose of the drug. A nebulizer may also be used. Nebulizers and spacers are equally effective in those with mild to moderate symptoms. However, insufficient evidence is available to determine whether a difference exists in those with severe disease.[227] For delivering short-acting beta-agonists in acute asthma in children, spacers may have advantages compared to nebulisers, but children with life-threatening asthma have not been studied.[228] There is no strong evidence for the use of intravenous LABA for adults or children who have acute asthma.[229] There is insufficient evidence to directly compare the effectiveness of a metered-dose inhaler attached to a homemade spacer compared to commercially available spacer for treating children with asthma.[230]
Adverse effects
Long-term use of inhaled corticosteroids at conventional doses carries a minor risk of adverse effects.[231] Risks include thrush, the development of cataracts, and a slightly slowed rate of growth.[231][232][233] Rinsing the mouth after the use of inhaled steroids can decrease the risk of thrush.[234] Higher doses of inhaled steroids may result in lower bone mineral density.[235]
Others
Inflammation in the lungs can be estimated by the level of exhaled nitric oxide.[236][237] The use of exhaled nitric oxide levels (FeNO) to guide asthma medication dosing may have small benefits for preventing asthma attacks but the potential benefits are not strong enough for this approach to be universally recommended as a method to guide asthma therapy in adults or children.[236][237]
When asthma is unresponsive to usual medications, other options are available for both emergency management and prevention of flareups. Additional options include:
- Humidified oxygen to alleviate hypoxia if saturations fall below 92%.[151]
- Corticosteroids by mouth, with five days of prednisone being the same two days of dexamethasone.[238] One review recommended a seven-day course of steroids.[239]
- Magnesium sulfate intravenous treatment increases bronchodilation when used in addition to other treatment in moderate severe acute asthma attacks.[17][240][241] In adults intravenous treatment results in a reduction of hospital admissions.[242] Low levels of evidence suggest that inhaled (nebulized) magnesium sulfate may have a small benefit for treating acute asthma in adults.[243] Overall, high-quality evidence do not indicate a large benefit for combining magnesium sulfate with standard inhaled treatments for adults with asthma.[243]
- Heliox, a mixture of helium and oxygen, may also be considered in severe unresponsive cases.[17]
- Intravenous salbutamol is not supported by available evidence and is thus used only in extreme cases.[244]
- Methylxanthines (such as theophylline) were once widely used, but do not add significantly to the effects of inhaled beta-agonists.[244] Their use in acute exacerbations is controversial.[245]
- The dissociative anaesthetic ketamine is theoretically useful if intubation and mechanical ventilation is needed in people who are approaching respiratory arrest; however, there is no evidence from clinical trials to support this.[246] A 2012 Cochrane review found no significant benefit from the use of ketamine in severe acute asthma in children.[247]
- For those with severe persistent asthma not controlled by inhaled corticosteroids and LABAs, bronchial thermoplasty may be an option.[248] It involves the delivery of controlled thermal energy to the airway wall during a series of bronchoscopies.[248][249] While it may increase exacerbation frequency in the first few months it appears to decrease the subsequent rate. Effects beyond one year are unknown.[250]
- Monoclonal antibody injections such as mepolizumab,[251] dupilumab,[252] or omalizumab may be useful in those with poorly controlled atopic asthma.[253] However, as of 2019[update] these medications are expensive and their use is therefore reserved for those with severe symptoms to achieve cost-effectiveness.[254] Monoclonal antibodies targeting interleukin-5 (IL-5) or its receptor (IL-5R), including mepolizumab, reslizumab or benralizumab, in addition to standard care in severe asthma is effective in reducing the rate of asthma exacerbations. There is limited evidence for improved health-related quality of life and lung function.[255]
- Evidence suggests that sublingual immunotherapy in those with both allergic rhinitis and asthma improve outcomes.[256]
- It is unclear if non-invasive positive pressure ventilation in children is of use as it has not been sufficiently studied.[257][needs update]
Adherence to asthma treatments
Staying with a treatment approach for preventing asthma exacerbations can be challenging, especially if the person is required to take medicine or treatments daily.[258] Reasons for low adherence range from a conscious decision to not follow the suggested medical treatment regime for various reasons including avoiding potential side effects, misinformation, or other beliefs about the medication.[258] Problems accessing the treatment and problems administering the treatment effectively can also result in lower adherence. Various approaches have been undertaken to try and improve adherence to treatments to help people prevent serious asthma exacerbations including digital interventions.[258]
Alternative medicine
Many people with asthma, like those with other chronic disorders, use alternative treatments; surveys show that roughly 50% use some form of unconventional therapy.[259][260] There is little data to support the effectiveness of most of these therapies.
Evidence is insufficient to support the usage of vitamin C or vitamin E for controlling asthma.[261][262] There is tentative support for use of vitamin C in exercise induced bronchospasm.[263] Fish oil dietary supplements (marine n-3 fatty acids)[264] and reducing dietary sodium[265] do not appear to help improve asthma control. In people with mild to moderate asthma, treatment with vitamin D supplementation or its hydroxylated metabolites does not reduce acute exacerbations or improve control.[266] There is no strong evidence to suggest that vitamin D supplements improve day-to-day asthma symptoms or a person's lung function.[266] There is no strong evidence to suggest that adults with asthma should avoid foods that contain monosodium glutamate (MSG).[267] There have not been enough high-quality studies performed to determine if children with asthma should avoid eating food that contains MSG.[267]
Acupuncture is not recommended for the treatment as there is insufficient evidence to support its use.[268][269] Air ionizers show no evidence that they improve asthma symptoms or benefit lung function; this applied equally to positive and negative ion generators.[270] Manual therapies, including osteopathic, chiropractic, physiotherapeutic and respiratory therapeutic manoeuvres, have insufficient evidence to support their use in treating asthma.[271] Pulmonary rehabilitation, however, may improve quality of life and functional exercise capacity when compared to usual care for adults with asthma.[272] The Buteyko breathing technique for controlling hyperventilation may result in a reduction in medication use; however, the technique does not have any effect on lung function.[157] Thus an expert panel felt that evidence was insufficient to support its use.[268] There is no clear evidence that breathing exercises are effective for treating children with asthma.[273]
Prognosis
The prognosis for asthma is generally good, especially for children with mild disease.[274] Mortality has decreased over the last few decades due to better recognition and improvement in care.[275] In 2010 the death rate was 170 per million for males and 90 per million for females.[276] Rates vary between countries by 100-fold.[276]
Globally it causes moderate or severe disability in 19.4 million people as of 2004[update] (16 million of which are in low and middle income countries).[277] Of asthma diagnosed during childhood, half of cases will no longer carry the diagnosis after a decade.[76] Airway remodelling is observed, but it is unknown whether these represent harmful or beneficial changes.[278] More recent data find that severe asthma can result in airway remodelling and the "asthma with chronic obstructive pulmonary disease syndrome (ACOS)" that has a poor prognosis.[279] Early treatment with corticosteroids seems to prevent or ameliorates a decline in lung function.[280] Asthma in children also has negative effects on quality of life of their parents.[281]
-
Asthma deaths per million persons in 20120–1011–1314–1718–2324–3233–4344–5051–6667–9596–251
-
Disability-adjusted life year for asthma per 100,000 inhabitants in 2004[282]no data0-100100–150150–200200–250250–300300–350350–400400–450450–500500–550550–600>600
Epidemiology

In 2019, approximately 262 million people worldwide were affected by asthma and approximately 461,000 people died from the disease.[8] Rates vary between countries with prevalences between 1 and 18%.[21] It is more common in developed than developing countries.[21] One thus sees lower rates in Asia, Eastern Europe and Africa.[20] Within developed countries it is more common in those who are economically disadvantaged while in contrast in developing countries it is more common in the affluent.[21] The reason for these differences is not well known.[21] Low- and middle-income countries make up more than 80% of the mortality.[284]
While asthma is twice as common in boys as girls,[21] severe asthma occurs at equal rates.[285] In contrast adult women have a higher rate of asthma than men[21] and it is more common in the young than the old.[20] In 2010, children with asthma experienced over 900,000 emergency department visits, making it the most common reason for admission to the hospital following an emergency department visit in the US in 2011.[286][287]
Global rates of asthma have increased significantly between the 1960s and 2008[18][288] with it being recognized as a major public health problem since the 1970s.[20] Rates of asthma have plateaued in the developed world since the mid-1990s with recent increases primarily in the developing world.[289] Asthma affects approximately 7% of the population of the United States[203] and 5% of people in the United Kingdom.[290] Canada, Australia and New Zealand have rates of about 14–15%.[291]
The average death rate from 2011 to 2015 from asthma in the UK was about 50% higher than the average for the European Union and had increased by about 5% in that time.[292] Children are more likely see a physician due to asthma symptoms after school starts in September.[293]
Population-based epidemiological studies describe temporal associations between acute respiratory illnesses, asthma, and development of severe asthma with irreversible airflow limitation (known as the asthma-chronic obstructive pulmonary disease "overlap" syndrome, or ACOS).[294][295][31] Additional prospective population-based data indicate that ACOS seems to represent a form of severe asthma, characterized by more frequent hospitalizations, and to be the result of early-onset asthma that has progressed to fixed airflow obstruction.[32]
Economics
From 2000 to 2010, the average cost per asthma-related hospital stay in the United States for children remained relatively stable at about $3,600, whereas the average cost per asthma-related hospital stay for adults increased from $5,200 to $6,600.[296] In 2010, Medicaid was the most frequent primary payer among children and adults aged 18–44 years in the United States; private insurance was the second most frequent payer.[296] Among both children and adults in the lowest income communities in the United States there is a higher rate of hospital stays for asthma in 2010 than those in the highest income communities.[296]
History

Asthma was recognized in ancient Egypt and was treated by drinking an incense mixture known as kyphi.[19] It was officially named as a specific respiratory problem by Hippocrates circa 450 BC, with the Greek word for "panting" forming the basis of our modern name.[20] In 200 BC it was believed to be at least partly related to the emotions.[28] In the 12th century the Jewish physician-philosopher Maimonides wrote a treatise on asthma in Arabic, based partly on Arabic sources, in which he discussed the symptoms, proposed various dietary and other means of treatment, and emphasized the importance of climate and clean air.[297] Traditional Chinese medicine also offered medication for asthma, as indicated by a surviving 14th-century manuscript curated by the Wellcome Foundation.[298]
In 1873, one of the first papers in modern medicine on the subject tried to explain the pathophysiology of the disease while one in 1872, concluded that asthma can be cured by rubbing the chest with chloroform liniment.[299][300] Medical treatment in 1880 included the use of intravenous doses of a drug called pilocarpine.[301]
In 1886, F. H. Bosworth theorized a connection between asthma and hay fever.[302]
At the beginning of the 20th century, the focus was the avoidance of allergens as well as selective beta-2 adrenoceptor agonists were used as treatment strategies.[303][304]
Epinephrine was first referred to in the treatment of asthma in 1905.[305] Oral corticosteroids began to be used for the condition in 1950. The use of a pressurized metered-dose inhaler was developed in the mid-1950s for the administration of adrenaline and isoproterenol and was later used as a beta2-adrenergic agonist.
Inhaled corticosteroids and selective short-acting beta agonists came into wide use in the 1960s.[306][307]
A well-documented case in the 19th century was that of young Theodore Roosevelt (1858–1919). At that time there was no effective treatment. Roosevelt's youth was in large part shaped by his poor health, partly related to his asthma. He experienced recurring nighttime asthma attacks that felt as if he was being smothered to death, terrifying the boy and his parents.[308]
During the 1930s to 1950s, asthma was known as one of the "holy seven" psychosomatic illnesses. Its cause was considered to be psychological, with treatment often based on psychoanalysis and other talking cures.[309] As these psychoanalysts interpreted the asthmatic wheeze as the suppressed cry of the child for its mother, they considered the treatment of depression to be especially important for individuals with asthma.[309]
In January 2021, an appeal court in France overturned a deportation order against a 40-year-old Bangladeshi man, who was a patient of asthma. His lawyers had argued that the dangerous levels of pollution in Bangladesh could possibly lead to worsening of his health condition, or even premature death.[310]
Notes
- ^ Jones D (2011). Roach P, Setter J, Esling J (eds.). Cambridge English Pronouncing Dictionary (18th ed.). Cambridge University Press. ISBN 978-0-521-15255-6.
- ^ Wells JC (2008). Longman Pronunciation Dictionary (3rd ed.). Longman. ISBN 978-1-4058-8118-0.
- ^ a b c d Drazen GM, Bel EH (2020). "81. Asthma". In Goldman L, Schafer AI (eds.). Goldman-Cecil Medicine. Vol. 1 (26th ed.). Philadelphia: Elsevier. pp. 527–535. ISBN 978-0-323-55087-1.
- ^ a b c d e f g h i j "Asthma Fact sheet №307". WHO. November 2013. Archived from the original on June 29, 2011. Retrieved March 3, 2016.
- ^ a b c Lemanske RF, Busse WW (February 2010). "Asthma: clinical expression and molecular mechanisms". The Journal of Allergy and Clinical Immunology. 125 (2 Suppl 2): S95-102. doi:10.1016/j.jaci.2009.10.047. PMC 2853245. PMID 20176271.
- ^ a b NHLBI Guideline 2007, pp. 169–72
- ^ a b c d e NHLBI Guideline 2007, p. 214
- ^ a b c d "Asthma–Level 3 cause" (PDF). The Lancet. 396: S108–S109. October 2020.
- ^ NHLBI Guideline 2007, pp. 11–12
- ^ GINA 2011, p. 20,51
- ^ a b c Yawn BP (September 2008). "Factors accounting for asthma variability: achieving optimal symptom control for individual patients" (PDF). Primary Care Respiratory Journal. 17 (3): 138–147. doi:10.3132/pcrj.2008.00004. PMC 6619889. PMID 18264646. Archived (PDF) from the original on March 26, 2009.
- ^ a b Kumar V, Abbas AK, Fausto N, Aster J (2010). Robbins and Cotran pathologic basis of disease (8th ed.). Saunders. p. 688. ISBN 978-1-4160-3121-5. OCLC 643462931.
- ^ Stedman's Medical Dictionary (28 ed.). Lippincott Williams & Wilkins. 2005. ISBN 978-0-7817-3390-8.
- ^ GINA 2011, p. 71
- ^ GINA 2011, p. 33
- ^ a b c d e Scott JP, Peters-Golden M (September 2013). "Antileukotriene agents for the treatment of lung disease". American Journal of Respiratory and Critical Care Medicine. 188 (5): 538–44. doi:10.1164/rccm.201301-0023PP. PMID 23822826.
- ^ a b c NHLBI Guideline 2007, pp. 373–75
- ^ a b Anandan C, Nurmatov U, van Schayck OC, Sheikh A (February 2010). "Is the prevalence of asthma declining? Systematic review of epidemiological studies". Allergy. 65 (2): 152–67. doi:10.1111/j.1398-9995.2009.02244.x. PMID 19912154. S2CID 19525219.
- ^ a b Manniche L (1999). Sacred luxuries: fragrance, aromatherapy, and cosmetics in ancient Egypt. Cornell University Press. pp. 49. ISBN 978-0-8014-3720-5.
- ^ a b c d e f g h i j k l m n Murray JF (2010). "Ch. 38 Asthma". In Mason RJ, Murray JF, Broaddus VC, Nadel JA, Martin TR, King Jr TE, Schraufnagel DE (eds.). Murray and Nadel's textbook of respiratory medicine (5th ed.). Elsevier. ISBN 978-1-4160-4710-0.
- ^ a b c d e f g h i GINA 2011, pp. 2–5
- ^ Jindal SK, ed. (2011). Textbook of pulmonary and critical care medicine. New Delhi: Jaypee Brothers Medical Publishers. p. 242. ISBN 978-93-5025-073-0. Archived from the original on April 24, 2016.
- ^ George RB (2005). Chest Medicine: Essentials of Pulmonary and Critical Care Medicine (5th ed.). Philadelphia: Lippincott Williams & Wilkins. p. 62. ISBN 978-0-7817-5273-2. Archived from the original on May 5, 2016.
- ^ British Guideline 2009, p. 14
- ^ GINA 2011, pp. 8–9
- ^ Boulet LP (April 2009). "Influence of Comorbid Conditions on Asthma". The European Respiratory Journal. 33 (4): 897–906. doi:10.1183/09031936.00121308. PMID 19336592.
- ^ Boulet LP, Boulay MÈ (June 2011). "Asthma-related comorbidities". Expert Review of Respiratory Medicine. 5 (3): 377–393. doi:10.1586/ers.11.34. PMID 21702660.
- ^ a b Harver A, Kotses H, eds. (2010). Asthma, Health and Society: A Public Health Perspective. New York: Springer. p. 315. ISBN 978-0-387-78285-0. Retrieved April 6, 2021.
- ^ Thomas M, Bruton A, Moffat M, Cleland J (September 2011). "Asthma and psychological dysfunction". Primary Care Respiratory Journal. 20 (3): 250–256. doi:10.4104/pcrj.2011.00058. PMC 6549858. PMID 21674122.
- ^ He X, Cheng G, He L, Liao B, Du Y, Xie X, et al. (January 2021). "Adults with current asthma but not former asthma have higher all-cause and cardiovascular mortality: a population-based prospective cohort study". Scientific Reports. 11 (1): 1329. Bibcode:2021NatSR..11.1329H. doi:10.1038/s41598-020-79264-4. PMC 7809422. PMID 33446724.
- ^ a b Silva GE, Sherrill DL, Guerra S, Barbee RA (July 2004). "Asthma as a risk factor for COPD in a longitudinal study". Chest. 126 (1): 59–65. doi:10.1378/chest.126.1.59. PMID 15249443.
- ^ a b de Marco R, Marcon A, Rossi A, Antó JM, Cerveri I, Gislason T, et al. (September 2015). "Asthma, COPD and overlap syndrome: a longitudinal study in young European adults". The European Respiratory Journal. 46 (3): 671–679. doi:10.1183/09031936.00008615. PMID 26113674. S2CID 2169875.
- ^ Gibson PG, McDonald VM (July 2015). "Asthma-COPD overlap 2015: now we are six". Thorax. 70 (7): 683–691. doi:10.1136/thoraxjnl-2014-206740. PMID 25948695. S2CID 38550372.
- ^ Thomsen HS, Webb JA, eds. (2014). Contrast media : safety issues and ESUR guidelines (Third ed.). Dordrecht: Springer. p. 54. ISBN 978-3-642-36724-3.
- ^ Agostini BA, Collares KF, Costa FD, Correa MB, Demarco FF (August 2019). "The role of asthma in caries occurrence – meta-analysis and meta-regression". The Journal of Asthma. 56 (8): 841–852. doi:10.1080/02770903.2018.1493602. PMID 29972654. S2CID 49694304.
- ^ a b Thomas MS, Parolia A, Kundabala M, Vikram M (June 2010). "Asthma and Oral Health: A Review". Australian Dental Journal. 55 (2): 128–133. doi:10.1111/j.1834-7819.2010.01226.x. PMID 20604752.
- ^ a b Martinez FD (January 2007). "Genes, environments, development and asthma: a reappraisal". The European Respiratory Journal. 29 (1): 179–84. doi:10.1183/09031936.00087906. PMID 17197483.
- ^ Miller RL, Ho SM (March 2008). "Environmental epigenetics and asthma: current concepts and call for studies". American Journal of Respiratory and Critical Care Medicine. 177 (6): 567–73. doi:10.1164/rccm.200710-1511PP. PMC 2267336. PMID 18187692.
- ^ Choudhry S, Seibold MA, Borrell LN, Tang H, Serebrisky D, Chapela R, et al. (July 2007). "Dissecting complex diseases in complex populations: asthma in latino americans". Proceedings of the American Thoracic Society. 4 (3): 226–33. doi:10.1513/pats.200701-029AW. PMC 2647623. PMID 17607004.
- ^ Dietert RR (September 2011). "Maternal and childhood asthma: risk factors, interactions, and ramifications". Reproductive Toxicology. 32 (2): 198–204. Bibcode:2011RepTx..32..198D. doi:10.1016/j.reprotox.2011.04.007. PMID 21575714.
- ^ Tan DJ, Walters EH, Perret JL, Lodge CJ, Lowe AJ, Matheson MC, Dharmage SC (February 2015). "Age-of-asthma onset as a determinant of different asthma phenotypes in adults: a systematic review and meta-analysis of the literature". Expert Review of Respiratory Medicine. 9 (1): 109–23. doi:10.1586/17476348.2015.1000311. PMID 25584929. S2CID 23213216.
- ^ Kelly FJ, Fussell JC (August 2011). "Air pollution and airway disease". Clinical and Experimental Allergy. 41 (8): 1059–71. doi:10.1111/j.1365-2222.2011.03776.x. PMID 21623970. S2CID 37717160.
- ^ "Occupational Asthmagens – New York State Department of Health".
- ^ "Occupational Asthmagens – HSE".
- ^ GINA 2011, p. 6
- ^ GINA 2011, p. 61
- ^ a b Gold DR, Wright R (2005). "Population disparities in asthma". Annual Review of Public Health. 26: 89–113. doi:10.1146/annurev.publhealth.26.021304.144528. PMID 15760282. S2CID 42988748.
- ^ American Lung Association (2001). "Urban Air Pollution and Health Inequities: A Workshop Report". Environmental Health Perspectives. 109 (s3): 357–374. doi:10.2307/3434783. ISSN 0091-6765. JSTOR 3434783. PMC 1240553. PMID 11427385.
- ^ Brooks N, Sethi R (February 1997). "The Distribution of Pollution: Community Characteristics and Exposure to Air Toxics". Journal of Environmental Economics and Management. 32 (2): 233–50. Bibcode:1997JEEM...32..233B. doi:10.1006/jeem.1996.0967.
- ^ McGwin G, Lienert J, Kennedy JI (March 2010). "Formaldehyde exposure and asthma in children: a systematic review". Environmental Health Perspectives. 118 (3): 313–7. doi:10.1289/ehp.0901143. PMC 2854756. PMID 20064771.
- ^ Jaakkola JJ, Knight TL (July 2008). "The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis". Environmental Health Perspectives. 116 (7): 845–53. doi:10.1289/ehp.10846. PMC 2453150. PMID 18629304.
- ^ Bornehag CG, Nanberg E (April 2010). "Phthalate exposure and asthma in children". International Journal of Andrology. 33 (2): 333–45. doi:10.1111/j.1365-2605.2009.01023.x. PMID 20059582.
- ^ Mamane A, Baldi I, Tessier JF, Raherison C, Bouvier G (June 2015). "Occupational exposure to pesticides and respiratory health". European Respiratory Review. 24 (136): 306–19. doi:10.1183/16000617.00006014. PMC 9487813. PMID 26028642.
- ^ Mamane A, Raherison C, Tessier JF, Baldi I, Bouvier G (September 2015). "Environmental exposure to pesticides and respiratory health". European Respiratory Review. 24 (137): 462–73. doi:10.1183/16000617.00006114. PMC 9487696. PMID 26324808.
- ^ Gruenwald T, Seals BA, Knibbs LD, Hosgood HD (December 2022). "Population Attributable Fraction of Gas Stoves and Childhood Asthma in the United States". International Journal of Environmental Research and Public Health. 20 (1): 75. doi:10.3390/ijerph20010075. PMC 9819315. PMID 36612391.
- ^ Heintze K, Petersen KU (June 2013). "The case of drug causation of childhood asthma: antibiotics and paracetamol". European Journal of Clinical Pharmacology. 69 (6): 1197–209. doi:10.1007/s00228-012-1463-7. PMC 3651816. PMID 23292157.
- ^ Henderson AJ, Shaheen SO (March 2013). "Acetaminophen and asthma". Paediatric Respiratory Reviews. 14 (1): 9–15, quiz 16. doi:10.1016/j.prrv.2012.04.004. PMID 23347656.
- ^ Cheelo M, Lodge CJ, Dharmage SC, Simpson JA, Matheson M, Heinrich J, Lowe AJ (January 2015). "Paracetamol exposure in pregnancy and early childhood and development of childhood asthma: a systematic review and meta-analysis". Archives of Disease in Childhood. 100 (1): 81–9. doi:10.1136/archdischild-2012-303043. PMID 25429049. S2CID 13520462.
- ^ van de Loo KF, van Gelder MM, Roukema J, Roeleveld N, Merkus PJ, Verhaak CM (January 2016). "Prenatal maternal psychological stress and childhood asthma and wheezing: a meta-analysis". The European Respiratory Journal. 47 (1): 133–46. doi:10.1183/13993003.00299-2015. PMID 26541526.
- ^ Ahluwalia SK, Matsui EC (April 2011). "The indoor environment and its effects on childhood asthma". Current Opinion in Allergy and Clinical Immunology. 11 (2): 137–43. doi:10.1097/ACI.0b013e3283445921. PMID 21301330. S2CID 35075329.
- ^ Arshad SH (January 2010). "Does exposure to indoor allergens contribute to the development of asthma and allergy?". Current Allergy and Asthma Reports. 10 (1): 49–55. doi:10.1007/s11882-009-0082-6. PMID 20425514. S2CID 30418306.
- ^ Custovic A, Simpson A (2012). "The role of inhalant allergens in allergic airways disease". Journal of Investigational Allergology & Clinical Immunology. 22 (6): 393–401, qiuz follow 401. PMID 23101182.
- ^ a b Gøtzsche PC, Johansen HK (April 2008). "House dust mite control measures for asthma". The Cochrane Database of Systematic Reviews. 2008 (2): CD001187. doi:10.1002/14651858.CD001187.pub3. PMC 8786269. PMID 18425868.
- ^ Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, Demoly P (July 2015). "Respiratory allergy caused by house dust mites: What do we really know?". The Journal of Allergy and Clinical Immunology. 136 (1): 38–48. doi:10.1016/j.jaci.2014.10.012. PMID 25457152.
- ^ Sauni R, Verbeek JH, Uitti J, Jauhiainen M, Kreiss K, Sigsgaard T (February 2015). "Remediating buildings damaged by dampness and mould for preventing or reducing respiratory tract symptoms, infections and asthma". The Cochrane Database of Systematic Reviews. 2015 (2): CD007897. doi:10.1002/14651858.CD007897.pub3. PMC 6769180. PMID 25715323.
- ^ a b NHLBI Guideline 2007, p. 11
- ^ Ramsey CD, Celedón JC (January 2005). "The hygiene hypothesis and asthma". Current Opinion in Pulmonary Medicine. 11 (1): 14–20. doi:10.1097/01.mcp.0000145791.13714.ae. PMID 15591883. S2CID 44556390.
- ^ Bufford JD, Gern JE (May 2005). "The hygiene hypothesis revisited". Immunology and Allergy Clinics of North America. 25 (2): 247–62, v–vi. doi:10.1016/j.iac.2005.03.005. PMID 15878454.
- ^ a b Brooks C, Pearce N, Douwes J (February 2013). "The hygiene hypothesis in allergy and asthma: an update". Current Opinion in Allergy and Clinical Immunology. 13 (1): 70–7. doi:10.1097/ACI.0b013e32835ad0d2. PMID 23103806. S2CID 23664343.
- ^ Rao D, Phipatanakul W (October 2011). "Impact of environmental controls on childhood asthma". Current Allergy and Asthma Reports. 11 (5): 414–20. doi:10.1007/s11882-011-0206-7. PMC 3166452. PMID 21710109.
- ^ Murk W, Risnes KR, Bracken MB (June 2011). "Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review". Pediatrics. 127 (6): 1125–38. doi:10.1542/peds.2010-2092. PMID 21606151. S2CID 26098640.
- ^ British Guideline 2009, p. 72
- ^ Neu J, Rushing J (June 2011). "Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis". Clinics in Perinatology. 38 (2): 321–31. doi:10.1016/j.clp.2011.03.008. PMC 3110651. PMID 21645799.
- ^ Von Hertzen LC, Haahtela T (February 2004). "Asthma and atopy – the price of affluence?". Allergy. 59 (2): 124–37. doi:10.1046/j.1398-9995.2003.00433.x. PMID 14763924. S2CID 34049674.
- ^ a b Martinez FD (July 2007). "CD14, endotoxin, and asthma risk: actions and interactions". Proceedings of the American Thoracic Society. 4 (3): 221–5. doi:10.1513/pats.200702-035AW. PMC 2647622. PMID 17607003.
- ^ a b c Elward G, Douglas KS (2010). Asthma. London: Manson Pub. pp. 27–29. ISBN 978-1-84076-513-7. Archived from the original on May 17, 2016.
- ^ a b c Ober C, Hoffjan S (March 2006). "Asthma genetics 2006: the long and winding road to gene discovery". Genes and Immunity. 7 (2): 95–100. doi:10.1038/sj.gene.6364284. PMID 16395390. S2CID 1887559.
- ^ Halapi E, Bjornsdottir US (January 2009). "Overview on the current status of asthma genetics". The Clinical Respiratory Journal. 3 (1): 2–7. doi:10.1111/j.1752-699X.2008.00119.x. PMID 20298365. S2CID 36471997.
- ^ a b Rapini RP, Bolognia JL, Jorizzo JL (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 978-1-4160-2999-1.
- ^ GINA 2011, p. 4
- ^ Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. (January 2013). "2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides". Arthritis and Rheumatism. 65 (1): 1–11. doi:10.1002/art.37715. PMID 23045170.
- ^ Beuther DA (January 2010). "Recent insight into obesity and asthma". Current Opinion in Pulmonary Medicine. 16 (1): 64–70. doi:10.1097/MCP.0b013e3283338fa7. PMID 19844182. S2CID 34157182.
- ^ Holguin F, Fitzpatrick A (March 2010). "Obesity, asthma, and oxidative stress". Journal of Applied Physiology. 108 (3): 754–9. doi:10.1152/japplphysiol.00702.2009. PMID 19926826.
- ^ Wood LG, Gibson PG (July 2009). "Dietary factors lead to innate immune activation in asthma". Pharmacology & Therapeutics. 123 (1): 37–53. doi:10.1016/j.pharmthera.2009.03.015. PMID 19375453.
- ^ O'Rourke ST (October 2007). "Antianginal actions of beta-adrenoceptor antagonists". American Journal of Pharmaceutical Education. 71 (5): 95. doi:10.5688/aj710595. PMC 2064893. PMID 17998992.
- ^ Salpeter S, Ormiston T, Salpeter E (2002). "Cardioselective beta-blockers for reversible airway disease". The Cochrane Database of Systematic Reviews. 2011 (4): CD002992. doi:10.1002/14651858.CD002992. PMC 8689715. PMID 12519582.
- ^ Morales DR, Jackson C, Lipworth BJ, Donnan PT, Guthrie B (April 2014). "Adverse respiratory effect of acute β-blocker exposure in asthma: a systematic review and meta-analysis of randomized controlled trials". Chest. 145 (4): 779–786. doi:10.1378/chest.13-1235. PMID 24202435.
- ^ Covar RA, Macomber BA, Szefler SJ (February 2005). "Medications as asthma triggers". Immunology and Allergy Clinics of North America. 25 (1): 169–90. doi:10.1016/j.iac.2004.09.009. PMID 15579370.
- ^ Lai T, Wu M, Liu J, Luo M, He L, Wang X, et al. (February 2018). "Acid-Suppressive Drug Use During Pregnancy and the Risk of Childhood Asthma: A Meta-analysis". Pediatrics. 141 (2): e20170889. doi:10.1542/peds.2017-0889. PMID 29326337.
- ^ a b c d Baxi SN, Phipatanakul W (April 2010). "The role of allergen exposure and avoidance in asthma". Adolescent Medicine. 21 (1): 57–71, viii–ix. PMC 2975603. PMID 20568555.
- ^ Sharpe RA, Bearman N, Thornton CR, Husk K, Osborne NJ (January 2015). "Indoor fungal diversity and asthma: a meta-analysis and systematic review of risk factors". The Journal of Allergy and Clinical Immunology. 135 (1): 110–22. doi:10.1016/j.jaci.2014.07.002. PMID 25159468.
- ^ Chen E, Miller GE (November 2007). "Stress and inflammation in exacerbations of asthma". Brain, Behavior, and Immunity. 21 (8): 993–9. doi:10.1016/j.bbi.2007.03.009. PMC 2077080. PMID 17493786.
- ^ Pike KC, Akhbari M, Kneale D, Harris KM (March 2018). "Interventions for autumn exacerbations of asthma in children". The Cochrane Database of Systematic Reviews. 2018 (3): CD012393. doi:10.1002/14651858.CD012393.pub2. PMC 6494188. PMID 29518252.
- ^ a b NHLBI Guideline 2007, p. 42
- ^ GINA 2011, p. 20
- ^ American Academy of Allergy, Asthma, and Immunology. "Five things physicians and patients should question" (PDF). Choosing Wisely. ABIM Foundation. Archived from the original (PDF) on November 3, 2012. Retrieved August 14, 2012.
- ^ Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute (US). 2007. 07-4051 – via NCBI.
- ^ Welsh EJ, Bara A, Barley E, Cates CJ (January 2010). Welsh EJ (ed.). "Caffeine for asthma" (PDF). The Cochrane Database of Systematic Reviews. 2010 (1): CD001112. doi:10.1002/14651858.CD001112.pub2. PMC 7053252. PMID 20091514.
- ^ NHLBI Guideline 2007, p. 58
- ^ Pinnock H, Shah R (April 2007). "Asthma". BMJ. 334 (7598): 847–50. doi:10.1136/bmj.39140.634896.BE. PMC 1853223. PMID 17446617.
- ^ NHLBI Guideline 2007, p. 59
- ^ a b Moore WC, Pascual RM (June 2010). "Update in asthma 2009". American Journal of Respiratory and Critical Care Medicine. 181 (11): 1181–7. doi:10.1164/rccm.201003-0321UP. PMC 3269238. PMID 20516492.
- ^ Harrison's principles of internal medicine (21st ed.). New York: McGraw Hill. 2022. p. 2150. ISBN 978-1-264-26850-4.
- ^ a b Self T, Chrisman C, Finch C (2009). "22. Asthma". In Koda-Kimble MA, Alldredge BK, et al. (eds.). Applied therapeutics: the clinical use of drugs (9th ed.). Philadelphia: Lippincott Williams & Wilkins. OCLC 230848069.
- ^ Delacourt C (June 2004). "[Bronchial changes in untreated asthma]" [Bronchial changes in untreated asthma]. Archives de Pédiatrie. 11 (Suppl 2): 71s–73s. doi:10.1016/S0929-693X(04)90003-6. PMID 15301800.
- ^ Schiffman G (December 18, 2009). "Chronic obstructive pulmonary disease". MedicineNet. Archived from the original on August 28, 2010. Retrieved September 2, 2010.
- ^ Gibson PG, McDonald VM (July 2015). "Asthma-COPD overlap 2015: now we are six". Thorax. 70 (7): 683–691. doi:10.1136/thoraxjnl-2014-206740. PMID 25948695. S2CID 38550372.
- ^ a b c d British Guideline 2009, p. 54
- ^ Current Review of Asthma. London: Current Medicine Group. 2003. p. 42. ISBN 978-1-4613-1095-2. Archived from the original on September 8, 2017.
- ^ Barnes PJ (2008). "Asthma". In Fauci AS, Braunwald E, Kasper DL (eds.). Harrison's Principles of Internal Medicine (17th ed.). New York: McGraw-Hill. pp. 1596–1607. ISBN 978-0-07-146633-2.
- ^ McMahon M (2011). Pediatrics a competency-based companion. Philadelphia: Saunders/Elsevier. ISBN 978-1-4160-5350-7.
- ^ Maitre B, Similowski T, Derenne JP (September 1995). "Physical examination of the adult patient with respiratory diseases: inspection and palpation". The European Respiratory Journal. 8 (9): 1584–93. doi:10.1183/09031936.95.08091584. PMID 8575588. S2CID 30677275. Archived from the original on April 29, 2015.
- ^ Werner HA (June 2001). "Status asthmaticus in children: a review". Chest. 119 (6): 1913–29. doi:10.1378/chest.119.6.1913. PMID 11399724.
- ^ a b Shiber JR, Santana J (May 2006). "Dyspnea". The Medical Clinics of North America. 90 (3): 453–79. doi:10.1016/j.mcna.2005.11.006. PMID 16473100.
- ^ a b Shah R, Saltoun CA (2012). "Chapter 14: Acute severe asthma (status asthmaticus)". Allergy and Asthma Proceedings. 33 (3): 47–50. doi:10.2500/aap.2012.33.3547. PMID 22794687.
- ^ a b Khan DA (January–February 2012). "Exercise-induced bronchoconstriction: burden and prevalence". Allergy and Asthma Proceedings. 33 (1): 1–6. doi:10.2500/aap.2012.33.3507. PMID 22370526.
- ^ Wuestenfeld JC, Wolfarth B (January 2013). "Special considerations for adolescent athletic and asthmatic patients". Open Access Journal of Sports Medicine. 4: 1–7. doi:10.2147/OAJSM.S23438. PMC 3871903. PMID 24379703.
- ^ GINA 2011, p. 17
- ^ Carlsen KH, Anderson SD, Bjermer L, Bonini S, Brusasco V, Canonica W, et al. (May 2008). "Treatment of exercise-induced asthma, respiratory and allergic disorders in sports and the relationship to doping: Part II of the report from the Joint Task Force of European Respiratory Society (ERS) and European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA(2)LEN". Allergy. 63 (5). European Respiratory, Society; European Academy of Allergy and Clinical, Immunology; GA(2)LEN: 492–505. doi:10.1111/j.1398-9995.2008.01663.x. PMID 18394123.
- ^ Kindermann W (2007). "Do inhaled beta(2)-agonists have an ergogenic potential in non-asthmatic competitive athletes?". Sports Medicine. 37 (2): 95–102. doi:10.2165/00007256-200737020-00001. PMID 17241101. S2CID 20993439.
- ^ Pluim BM, de Hon O, Staal JB, Limpens J, Kuipers H, Overbeek SE, et al. (January 2011). "β₂-Agonists and physical performance: a systematic review and meta-analysis of randomized controlled trials". Sports Medicine. 41 (1): 39–57. doi:10.2165/11537540-000000000-00000. PMID 21142283. S2CID 189906919.
- ^ a b c Baur X, Aasen TB, Burge PS, Heederik D, Henneberger PK, Maestrelli P, et al. (June 2012). "The management of work-related asthma guidelines: a broader perspective". European Respiratory Review. 21 (124). ERS Task Force on the Management of Work-related, Asthma: 125–39. doi:10.1183/09059180.00004711. PMC 9487296. PMID 22654084.
- ^ Kunnamo I, ed. (2005). Evidence-based medicine guidelines. Chichester: Wiley. p. 214. ISBN 978-0-470-01184-3.
- ^ Frew AJ (2008). "Chapter 42: Occupational Asthma". In Castro M, Kraft M (eds.). Clinical Asthma. Philadelphia: Mosby / Elsevier. ISBN 978-0-323-07081-2.
- ^ Chang JE, White A, Simon RA, Stevenson DD (2012). "Aspirin-exacerbated respiratory disease: burden of disease". Allergy and Asthma Proceedings. 33 (2): 117–21. doi:10.2500/aap.2012.33.3541. PMID 22525387.
- ^ "Aspirin Exacerbated Respiratory Disease (AERD)". aaaai.org. American Academy of Allergy Asthma & Immunology. August 3, 2018. Archived from the original on September 18, 2018. Retrieved August 2, 2018.
- ^ Kennedy JL, Stoner AN, Borish L (November 2016). "Aspirin-exacerbated respiratory disease: Prevalence, diagnosis, treatment, and considerations for the future". American Journal of Rhinology & Allergy. 30 (6): 407–413. doi:10.2500/ajra.2016.30.4370. PMC 5108840. PMID 28124651.
- ^ a b c Adams KE, Rans TS (December 2013). "Adverse reactions to alcohol and alcoholic beverages". Annals of Allergy, Asthma & Immunology. 111 (6): 439–45. doi:10.1016/j.anai.2013.09.016. PMID 24267355.
- ^ Peters SP (2014). "Asthma phenotypes: nonallergic (intrinsic) asthma". The Journal of Allergy and Clinical Immunology. In Practice. 2 (6): 650–652. doi:10.1016/j.jaip.2014.09.006. PMID 25439352.
- ^ Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG (February 1989). "Association of asthma with serum IgE levels and skin-test reactivity to allergens". The New England Journal of Medicine. 320 (5): 271–277. doi:10.1056/NEJM198902023200502. PMID 2911321.
- ^ Hahn DL (2021). "Chlamydia pneumoniae and chronic asthma: Updated systematic review and meta-analysis of population attributable risk". PLOS ONE. 16 (4): e0250034. Bibcode:2021PLoSO..1650034H. doi:10.1371/journal.pone.0250034. PMC 8055030. PMID 33872336.
- ^ Hahn DL, Schultek NM (2022). "Infectious Asthma: An Easily Identified Clinical Presentation with Implications for Diagnosis, Prognosis, Treatment, and Prevention of Asthma". Journal of Asthma and Allergy. 15: 1269–1272. doi:10.2147/JAA.S379890. PMC 9508995. PMID 36164333.
- ^ a b Hahn DL (August 1995). "Infectious asthma: a reemerging clinical entity?". The Journal of Family Practice. 41 (2): 153–157. PMID 7636455.
- ^ Hahn DL, Peeling RW, Dillon E, McDonald R, Saikku P (February 2000). "Serologic markers for Chlamydia pneumoniae in asthma". Annals of Allergy, Asthma & Immunology. 84 (2): 227–233. doi:10.1016/S1081-1206(10)62760-3. PMID 10719781.
- ^ a b Wagshul FA, Brown DT, Schultek NM, Hahn DL (2021). "Outcomes of Antibiotics in Adults with 'Difficult to Treat' Asthma or the Overlap Syndrome". Journal of Asthma and Allergy. 14: 703–712. doi:10.2147/JAA.S313480. PMC 8216074. PMID 34163182.
- ^ Rantala A, Jaakkola JJ, Jaakkola MS (2011). "Respiratory infections precede adult-onset asthma". PLOS ONE. 6 (12): e27912. Bibcode:2011PLoSO...627912R. doi:10.1371/journal.pone.0027912. PMC 3244385. PMID 22205932.
- ^ Kuruvilla ME, Lee FE, Lee GB (April 2019). "Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease". Clinical Reviews in Allergy & Immunology. 56 (2): 219–233. doi:10.1007/s12016-018-8712-1. PMC 6411459. PMID 30206782.
- ^ Ray A, Camiolo M, Fitzpatrick A, Gauthier M, Wenzel SE (July 2020). "Are We Meeting the Promise of Endotypes and Precision Medicine in Asthma?". Physiological Reviews. 100 (3): 983–1017. doi:10.1152/physrev.00023.2019. PMC 7474260. PMID 31917651.
- ^ a b NHLBI Guideline 2007, p. 46
- ^ Lichtenstein R (2013). Pediatric emergencies. Philadelphia: Elsevier. p. 1022. ISBN 978-0-323-22733-9. Archived from the original on September 8, 2017.
- ^ Van Bever HP, Han E, Shek L, Yi Chng S, Goh D (November 2010). "An approach to preschool wheezing: to label as asthma?". The World Allergy Organization Journal. 3 (11): 253–257. doi:10.1097/WOX.0b013e3181fc7fa1. PMC 3651058. PMID 23282943.
- ^ Gibson PG, McDonald VM, Marks GB (September 2010). "Asthma in older adults". Lancet. 376 (9743): 803–13. doi:10.1016/S0140-6736(10)61087-2. PMID 20816547. S2CID 12275555.
- ^ Hargreave FE, Parameswaran K (August 2006). "Asthma, COPD and bronchitis are just components of airway disease". The European Respiratory Journal. 28 (2): 264–7. doi:10.1183/09031936.06.00056106. PMID 16880365.
- ^ Diaz PK (2009). "23. Chronic obstructive pulmonary disease". Applied therapeutics: the clinical use of drugs (9th ed.). Philadelphia: Lippincott Williams & Wilkins.
- ^ a b NHLBI Guideline 2007, pp. 184–85
- ^ "Asthma". World Health Organization. April 2017. Archived from the original on June 29, 2011. Retrieved May 30, 2017.
- ^ Henneberger PK (April 2007). "Work-exacerbated asthma". Current Opinion in Allergy and Clinical Immunology. 7 (2): 146–51. doi:10.1097/ACI.0b013e328054c640. PMID 17351467. S2CID 20728967.
- ^ Lodge CJ, Allen KJ, Lowe AJ, Hill DJ, Hosking CS, Abramson MJ, Dharmage SC (2012). "Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: a systematic review of longitudinal studies". Clinical & Developmental Immunology. 2012: 176484. doi:10.1155/2012/176484. PMC 3251799. PMID 22235226.
- ^ Chen CM, Tischer C, Schnappinger M, Heinrich J (January 2010). "The role of cats and dogs in asthma and allergy—a systematic review". International Journal of Hygiene and Environmental Health. 213 (1): 1–31. Bibcode:2010IJHEH.213....1C. doi:10.1016/j.ijheh.2009.12.003. PMID 20053584.
- ^ a b Prescott SL, Tang ML (May 2005). "The Australasian Society of Clinical Immunology and Allergy position statement: Summary of allergy prevention in children". The Medical Journal of Australia. 182 (9). Australasian Society of Clinical Immunology and, Allergy: 464–7. doi:10.5694/j.1326-5377.2005.tb06787.x. PMID 15865590. S2CID 8172491.
- ^ a b c d e f g h i j k Bertrand P, Faccin AB (January 31, 2020). "Asthma: Treatment". In Bertrand P, Sánchez I (eds.). Pediatric Respiratory Diseases: A Comprehensive Textbook. Springer Nature. pp. 415–428. doi:10.1007/978-3-030-26961-6. ISBN 978-3-03-026961-6. S2CID 210985844.
- ^ Cates CJ, Rowe BH (February 2013). "Vaccines for preventing influenza in people with asthma". The Cochrane Database of Systematic Reviews. 2 (2): CD000364. doi:10.1002/14651858.CD000364.pub4. PMC 6999427. PMID 23450529.
- ^ "Strategic Advisory Group of Experts on Immunization - report of the extraordinary meeting on the influenza A (H1N1) 2009 pandemic, 7 July 2009". Relevé Épidémiologique Hebdomadaire. 84 (30): 301–4. July 2009. PMID 19630186.
- ^ a b Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A (May 2014). "Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis". Lancet. 383 (9928): 1549–60. doi:10.1016/S0140-6736(14)60082-9. PMID 24680633. S2CID 8532979.
- ^ Ripoll BC, Leutholtz I (2011). Exercise and disease management (2nd ed.). Boca Raton: CRC Press. p. 100. ISBN 978-1-4398-2759-8. Archived from the original on May 6, 2016.
- ^ a b c d e NHLBI Guideline 2007, p. 213
- ^ a b "British Guideline on the Management of Asthma" (PDF). Scottish Intercollegiate Guidelines Network. 2008. Archived (PDF) from the original on August 19, 2008. Retrieved August 4, 2008.
- ^ Rabe KF, Adachi M, Lai CK, Soriano JB, Vermeire PA, Weiss KB, Weiss ST (July 2004). "Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys". The Journal of Allergy and Clinical Immunology. 114 (1): 40–47. doi:10.1016/j.jaci.2004.04.042. PMID 15241342.
- ^ Demoly P, Gueron B, Annunziata K, Adamek L, Walters RD (June 2010). "Update on asthma control in five European countries: results of a 2008 survey". European Respiratory Review. 19 (116): 150–157. doi:10.1183/09059180.00002110. PMC 9682581. PMID 20956184. S2CID 13408225.
- ^ FitzGerald JM, Boulet LP, McIvor RA, Zimmerman S, Chapman KR (2006). "Asthma control in Canada remains suboptimal: the Reality of Asthma Control (TRAC) study". Canadian Respiratory Journal. 13 (5): 253–259. doi:10.1155/2006/753083. PMC 2683303. PMID 16896426.
- ^ Herland K, Akselsen JP, Skjønsberg OH, Bjermer L (January 2005). "How representative are clinical study patients with asthma or COPD for a larger 'real life' population of patients with obstructive lung disease?". Respiratory Medicine. 99 (1): 11–19. doi:10.1016/j.rmed.2004.03.026. PMID 15672843.
- ^ Travers J, Marsh S, Williams M, Weatherall M, Caldwell B, Shirtcliffe P, et al. (March 2007). "External validity of randomised controlled trials in asthma: to whom do the results of the trials apply?". Thorax. 62 (3): 219–223. doi:10.1136/thx.2006.066837. PMC 2117157. PMID 17105779.
- ^ Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, et al. (April 2007). "Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma". American Journal of Respiratory and Critical Care Medicine. 175 (8): 783–790. doi:10.1164/rccm.200511-1746OC. PMC 1899291. PMID 17204725.
- ^ Stapleton M, Howard-Thompson A, George C, Hoover RM, Self TH (2011). "Smoking and asthma". Journal of the American Board of Family Medicine. 24 (3): 313–322. doi:10.3122/jabfm.2011.03.100180. PMID 21551404. S2CID 3183714.
- ^ Hayes CE, Nuss HJ, Tseng TS, Moody-Thomas S (2015). "Use of asthma control indicators in measuring inhaled corticosteroid effectiveness in asthmatic smokers: a systematic review". The Journal of Asthma. 52 (10): 996–1005. doi:10.3109/02770903.2015.1065422. PMID 26418843. S2CID 36916271.
- ^ a b c Kew KM, Nashed M, Dulay V, Yorke J (September 2016). "Cognitive behavioural therapy (CBT) for adults and adolescents with asthma". The Cochrane Database of Systematic Reviews. 2016 (9): CD011818. doi:10.1002/14651858.CD011818.pub2. PMC 6457695. PMID 27649894.
- ^ Paudyal P, Hine P, Theadom A, Apfelbacher CJ, Jones CJ, Yorke J, et al. (May 2014). "Written emotional disclosure for asthma". The Cochrane Database of Systematic Reviews (5): CD007676. doi:10.1002/14651858.CD007676.pub2. PMC 11254376. PMID 24842151.
- ^ Bhogal S, Zemek R, Ducharme FM (July 2006). "Written action plans for asthma in children". The Cochrane Database of Systematic Reviews (3): CD005306. doi:10.1002/14651858.CD005306.pub2. PMID 16856090.
- ^ McCallum GB, Morris PS, Brown N, Chang AB (August 2017). "Culture-specific programs for children and adults from minority groups who have asthma". The Cochrane Database of Systematic Reviews. 2017 (8): CD006580. doi:10.1002/14651858.CD006580.pub5. PMC 6483708. PMID 28828760.
- ^ Kew KM, Carr R, Donovan T, Gordon M (April 2017). "Asthma education for school staff". The Cochrane Database of Systematic Reviews. 2017 (4): CD012255. doi:10.1002/14651858.CD012255.pub2. PMC 6478185. PMID 28402017.
- ^ Welsh EJ, Hasan M, Li P (October 2011). "Home-based educational interventions for children with asthma". The Cochrane Database of Systematic Reviews. 2014 (10): CD008469. doi:10.1002/14651858.CD008469.pub2. PMC 8972064. PMID 21975783.
- ^ Yorke J, Shuldham C (April 2005). "Family therapy for chronic asthma in children". The Cochrane Database of Systematic Reviews. 2005 (2): CD000089. doi:10.1002/14651858.CD000089.pub2. PMC 7038646. PMID 15846599.
- ^ Harris K, Kneale D, Lasserson TJ, McDonald VM, Grigg J, Thomas J, et al. (Cochrane Airways Group) (January 2019). "School-based self-management interventions for asthma in children and adolescents: a mixed methods systematic review". The Cochrane Database of Systematic Reviews. 1 (1): CD011651. doi:10.1002/14651858.CD011651.pub2. PMC 6353176. PMID 30687940.
- ^ Kew KM, Malik P, Aniruddhan K, Normansell R (October 2017). "Shared decision-making for people with asthma". The Cochrane Database of Systematic Reviews. 2017 (10): CD012330. doi:10.1002/14651858.CD012330.pub2. PMC 6485676. PMID 28972652.
- ^ Gatheral TL, Rushton A, Evans DJ, Mulvaney CA, Halcovitch NR, Whiteley G, et al. (April 2017). "Personalised asthma action plans for adults with asthma". The Cochrane Database of Systematic Reviews. 2017 (4): CD011859. doi:10.1002/14651858.CD011859.pub2. PMC 6478068. PMID 28394084.
- ^ Welsh EJ, Carr R, et al. (Cochrane Airways Group) (September 2015). "Pulse oximeters to self monitor oxygen saturation levels as part of a personalised asthma action plan for people with asthma". The Cochrane Database of Systematic Reviews. 2015 (9): CD011584. doi:10.1002/14651858.CD011584.pub2. PMC 9426972. PMID 26410043.
- ^ NHLBI Guideline 2007, p. 69
- ^ Thomson NC, Spears M (February 2005). "The influence of smoking on the treatment response in patients with asthma". Current Opinion in Allergy and Clinical Immunology. 5 (1): 57–63. doi:10.1097/00130832-200502000-00011. PMID 15643345. S2CID 25065026.
- ^ Stapleton M, Howard-Thompson A, George C, Hoover RM, Self TH (2011). "Smoking and asthma". Journal of the American Board of Family Medicine. 24 (3): 313–322. doi:10.3122/jabfm.2011.03.100180. PMID 21551404.
- ^ Singh M, Jaiswal N (June 2013). "Dehumidifiers for chronic asthma". The Cochrane Database of Systematic Reviews. 2014 (6): CD003563. doi:10.1002/14651858.CD003563.pub2. PMC 10646756. PMID 23760885.
- ^ Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ (September 2013). "Physical training for asthma". The Cochrane Database of Systematic Reviews. 9 (9): CD001116. doi:10.1002/14651858.CD001116.pub4. PMID 24085631.
- ^ Yang ZY, Zhong HB, Mao C, Yuan JQ, Huang YF, Wu XY, et al. (April 2016). "Yoga for asthma". The Cochrane Database of Systematic Reviews. 4 (11): CD010346. doi:10.1002/14651858.cd010346.pub2. PMC 6880926. PMID 27115477.
- ^ Adeniyi FB, Young T (July 2012). "Weight loss interventions for chronic asthma". The Cochrane Database of Systematic Reviews (7): CD009339. doi:10.1002/14651858.CD009339.pub2. PMID 22786526.
- ^ Cheng J, Pan T, Ye GH, Liu Q (July 2005). "Calorie controlled diet for chronic asthma". The Cochrane Database of Systematic Reviews (3): CD004674. doi:10.1002/14651858.CD004674.pub2. PMID 16034941.
- ^ Almahayni O, Hammond L (March 13, 2024). "Does the Wim Hof Method have a beneficial impact on physiological and psychological outcomes in healthy and non-healthy participants? A systematic review". PLOS ONE. 19 (3): e0286933. Bibcode:2024PLoSO..1986933A. doi:10.1371/journal.pone.0286933. ISSN 1932-6203. PMC 10936795. PMID 38478473.
- ^ "QRG 153 • British guideline on the management of asthma" (PDF). SIGN. September 2016. Archived (PDF) from the original on October 9, 2016. Retrieved October 6, 2016.
- ^ Normansell R, Sayer B, Waterson S, Dennett EJ, Del Forno M, Dunleavy A (June 2018). "Antibiotics for exacerbations of asthma". The Cochrane Database of Systematic Reviews. 2018 (6): CD002741. doi:10.1002/14651858.CD002741.pub2. PMC 6513273. PMID 29938789.
- ^ Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, et al. (May 2013). "An Official American Thoracic Society Clinical Practice Guideline: Exercise-Induced Bronchoconstriction". American Journal of Respiratory and Critical Care Medicine. 187 (9): 1016–1027. doi:10.1164/rccm.201303-0437ST. PMID 23634861.
- ^ a b Griffiths B, Ducharme FM (August 2013). "Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children". The Cochrane Database of Systematic Reviews (8): CD000060. doi:10.1002/14651858.CD000060.pub2. PMID 23966133.
- ^ a b Kirkland SW, Vandenberghe C, Voaklander B, Nikel T, Campbell S, Rowe BH (January 2017). "Combined inhaled beta-agonist and anticholinergic agents for emergency management in adults with asthma". The Cochrane Database of Systematic Reviews. 1 (1): CD001284. doi:10.1002/14651858.CD001284.pub2. PMC 6465060. PMID 28076656.
- ^ Vézina K, Chauhan BF, Ducharme FM (July 2014). "Inhaled anticholinergics and short-acting beta(2)-agonists versus short-acting beta2-agonists alone for children with acute asthma in hospital". The Cochrane Database of Systematic Reviews. 2014 (7): CD010283. doi:10.1002/14651858.CD010283.pub2. PMC 10772940. PMID 25080126.
- ^ Teoh L, Cates CJ, Hurwitz M, Acworth JP, van Asperen P, Chang AB (April 2012). "Anticholinergic therapy for acute asthma in children" (PDF). The Cochrane Database of Systematic Reviews (4): CD003797. doi:10.1002/14651858.CD003797.pub2. PMC 11329281. PMID 22513916.
- ^ Rodrigo GJ, Nannini LJ (March 2006). "Comparison between nebulized adrenaline and beta2 agonists for the treatment of acute asthma. A meta-analysis of randomized trials". The American Journal of Emergency Medicine. 24 (2): 217–22. doi:10.1016/j.ajem.2005.10.008. PMID 16490653.
- ^ NHLBI Guideline 2007, p. 351
- ^ Smith M, Iqbal S, Elliott TM, Everard M, Rowe BH (2003). "Corticosteroids for hospitalised children with acute asthma". The Cochrane Database of Systematic Reviews. 2003 (2): CD002886. doi:10.1002/14651858.CD002886. PMC 6999806. PMID 12804441.
- ^ Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW (2001). "Early emergency department treatment of acute asthma with systemic corticosteroids". The Cochrane Database of Systematic Reviews (1): CD002178. doi:10.1002/14651858.CD002178. PMC 7025797. PMID 11279756.
- ^ Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW (July 2007). "Corticosteroids for preventing relapse following acute exacerbations of asthma". The Cochrane Database of Systematic Reviews (3): CD000195. doi:10.1002/14651858.CD000195.pub2. PMID 17636617. S2CID 11992578.
- ^ a b Nair P, Milan SJ, Rowe BH (December 2012). "Addition of intravenous aminophylline to inhaled beta(2)-agonists in adults with acute asthma". The Cochrane Database of Systematic Reviews. 2012 (12): CD002742. doi:10.1002/14651858.CD002742.pub2. PMC 7093892. PMID 23235591.
- ^ NHLBI Guideline 2007, p. 218
- ^ a b Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ (May 2010). Ducharme FM (ed.). "Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children". The Cochrane Database of Systematic Reviews (5): CD005535. doi:10.1002/14651858.CD005535.pub2. PMC 4169792. PMID 20464739.
- ^ a b Ni Chroinin M, Greenstone I, Lasserson TJ, Ducharme FM (October 2009). "Addition of inhaled long-acting beta2-agonists to inhaled steroids as first line therapy for persistent asthma in steroid-naive adults and children". The Cochrane Database of Systematic Reviews (4): CD005307. doi:10.1002/14651858.CD005307.pub2. PMC 4170786. PMID 19821344.
- ^ Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ (April 2010). Ducharme FM (ed.). "Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma". The Cochrane Database of Systematic Reviews (4): CD005533. doi:10.1002/14651858.CD005533.pub2. PMC 4169793. PMID 20393943.
- ^ a b Fanta CH (March 2009). "Asthma". The New England Journal of Medicine. 360 (10): 1002–14. doi:10.1056/NEJMra0804579. PMID 19264689.
- ^ Cates CJ, Cates MJ (April 2012). Cates CJ (ed.). "Regular treatment with formoterol for chronic asthma: serious adverse events". The Cochrane Database of Systematic Reviews. 4 (4): CD006923. doi:10.1002/14651858.CD006923.pub3. PMC 4017186. PMID 22513944.
- ^ Cates CJ, Cates MJ (July 2008). Cates CJ (ed.). "Regular treatment with salmeterol for chronic asthma: serious adverse events". The Cochrane Database of Systematic Reviews (3): CD006363. doi:10.1002/14651858.CD006363.pub2. PMC 4015854. PMID 18646149.
- ^ a b Chauhan BF, Chartrand C, Ni Chroinin M, Milan SJ, Ducharme FM (November 2015). "Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children". The Cochrane Database of Systematic Reviews. 2015 (11): CD007949. doi:10.1002/14651858.CD007949.pub2. PMC 4167878. PMID 26594816.
- ^ Chauhan BF, Ducharme FM (January 2014). "Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma" (PDF). The Cochrane Database of Systematic Reviews. 2014 (1): CD003137. doi:10.1002/14651858.CD003137.pub5. PMC 10514761. PMID 24459050.
- ^ a b Chauhan BF, Jeyaraman MM, Singh Mann A, Lys J, Abou-Setta AM, Zarychanski R, Ducharme FM (March 2017). "Addition of anti-leukotriene agents to inhaled corticosteroids for adults and adolescents with persistent asthma". The Cochrane Database of Systematic Reviews. 3 (4): CD010347. doi:10.1002/14651858.CD010347.pub2. PMC 6464690. PMID 28301050.
- ^ Watts K, Chavasse RJ (May 2012). Watts K (ed.). "Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children". The Cochrane Database of Systematic Reviews. 5 (5): CD006100. doi:10.1002/14651858.CD006100.pub2. PMC 7387678. PMID 22592708.
- ^ Miligkos M, Bannuru RR, Alkofide H, Kher SR, Schmid CH, Balk EM (November 2015). "Leukotriene-receptor antagonists versus placebo in the treatment of asthma in adults and adolescents: a systematic review and meta-analysis". Annals of Internal Medicine. 163 (10): 756–67. doi:10.7326/M15-1059. PMC 4648683. PMID 26390230.
- ^ British Guideline 2009, p. 43
- ^ Chauhan BF, Ben Salah R, Ducharme FM (October 2013). "Addition of anti-leukotriene agents to inhaled corticosteroids in children with persistent asthma". The Cochrane Database of Systematic Reviews (10): CD009585. doi:10.1002/14651858.CD009585.pub2. PMC 4235447. PMID 24089325.
- ^ "Zyflo (Zileuton tablets)" (PDF). United States Food and Drug Administration. Cornerstone Therapeutics Inc. June 2012. p. 1. Archived (PDF) from the original on December 13, 2014. Retrieved December 12, 2014.
- ^ Solèr M (September 2001). "Omalizumab, a monoclonal antibody against IgE for the treatment of allergic diseases". International Journal of Clinical Practice. 55 (7): 480–483. doi:10.1111/j.1742-1241.2001.tb11095.x. PMID 11594260. S2CID 41311909.
- ^ McDonald NJ, Bara AI (2003). "Anticholinergic therapy for chronic asthma in children over two years of age". The Cochrane Database of Systematic Reviews. 2014 (3): CD003535. doi:10.1002/14651858.CD003535. PMC 8717339. PMID 12917970.
- ^ Westby M, Benson M, Gibson P (2004). "Anticholinergic agents for chronic asthma in adults". The Cochrane Database of Systematic Reviews. 2017 (3): CD003269. doi:10.1002/14651858.CD003269.pub2. PMC 6483359. PMID 15266477.
- ^ Dean T, Dewey A, Bara A, Lasserson TJ, Walters EH (2003). "Chloroquine as a steroid sparing agent for asthma". The Cochrane Database of Systematic Reviews (4): CD003275. doi:10.1002/14651858.CD003275. PMID 14583965.
- ^ Davies H, Olson L, Gibson P (2000). "Methotrexate as a steroid sparing agent for asthma in adults". The Cochrane Database of Systematic Reviews. 1998 (2): CD000391. doi:10.1002/14651858.CD000391. PMC 6483672. PMID 10796540.
- ^ Hiles SA, McDonald VM, Guilhermino M, Brusselle GG, Gibson PG (November 2019). "Does maintenance azithromycin reduce asthma exacerbations? An individual participant data meta-analysis". The European Respiratory Journal. 54 (5). doi:10.1183/13993003.01381-2019. PMID 31515407. S2CID 202567597.
- ^ GINA. "Difficult-to-Treat and Severe Asthma in Adolescent and Adult Patients: Diagnosis and Management". Global Initiative for Asthma. Retrieved August 1, 2021.
- ^ Steel HC, Theron AJ, Cockeran R, Anderson R, Feldman C (2012). "Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics". Mediators of Inflammation. 2012: 584262. doi:10.1155/2012/584262. PMC 3388425. PMID 22778497.
- ^ Hahn DL (December 2019). "When guideline treatment of asthma fails, consider a macrolide antibiotic". The Journal of Family Practice. 68 (10): 536, 540, 542, 545. PMID 31860697.
- ^ Lee B, Man KK, Wong E, Tan T, Sheikh A, Bloom CI (November 18, 2024). "Antidiabetic Medication and Asthma Attacks". JAMA Internal Medicine. doi:10.1001/jamainternmed.2024.5982. ISSN 2168-6106.
- ^ Mundell E (November 18, 2024). "Diabetes Meds Metformin, GLP-1s Can Also Curb Asthma". www.healthday.com. Retrieved November 20, 2024.
- ^ Kew KM, Beggs S, Ahmad S (May 2015). "Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids". The Cochrane Database of Systematic Reviews. 2017 (5): CD011316. doi:10.1002/14651858.CD011316.pub2. PMC 6486153. PMID 25997166.
- ^ a b c Ahmad S, Kew KM, Normansell R (June 2015). "Stopping long-acting beta2-agonists (LABA) for adults with asthma well controlled by LABA and inhaled corticosteroids" (PDF). The Cochrane Database of Systematic Reviews. 2015 (6): CD011306. doi:10.1002/14651858.CD011306.pub2. PMC 11114094. PMID 26089258.
- ^ NHLBI Guideline 2007, p. 250
- ^ Cates CJ, Welsh EJ, Rowe BH, et al. (Cochrane Airways Group) (September 2013). "Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma". The Cochrane Database of Systematic Reviews. 2013 (9): CD000052. doi:10.1002/14651858.CD000052.pub3. PMC 7032675. PMID 24037768.
- ^ Travers AH, Milan SJ, Jones AP, Camargo CA, Rowe BH (December 2012). "Addition of intravenous beta(2)-agonists to inhaled beta(2)-agonists for acute asthma". The Cochrane Database of Systematic Reviews. 2012 (12): CD010179. doi:10.1002/14651858.CD010179. PMC 11289706. PMID 23235685.
- ^ Rodriguez C, Sossa M, Lozano JM (April 2008). "Commercial versus home-made spacers in delivering bronchodilator therapy for acute therapy in children". The Cochrane Database of Systematic Reviews. 2017 (2): CD005536. doi:10.1002/14651858.CD005536.pub2. PMC 6483735. PMID 18425921.
- ^ a b Rachelefsky G (January 2009). "Inhaled corticosteroids and asthma control in children: assessing impairment and risk". Pediatrics. 123 (1): 353–66. doi:10.1542/peds.2007-3273. PMID 19117903. S2CID 22386752.
- ^ Dahl R (August 2006). "Systemic side effects of inhaled corticosteroids in patients with asthma". Respiratory Medicine. 100 (8): 1307–17. doi:10.1016/j.rmed.2005.11.020. PMID 16412623.
- ^ Thomas MS, Parolia A, Kundabala M, Vikram M (June 2010). "Asthma and oral health: a review". Australian Dental Journal. 55 (2): 128–33. doi:10.1111/j.1834-7819.2010.01226.x. PMID 20604752.
- ^ Domino FJ, Baldor RA, Golding J, Grimes JA (2014). The 5-Minute Clinical Consult Premium 2015. Lippincott Williams & Wilkins. p. 192. ISBN 978-1-4511-9215-5.
- ^ Skoner DP (December 2016). "Inhaled corticosteroids: Effects on growth and bone health". Annals of Allergy, Asthma & Immunology. 117 (6): 595–600. doi:10.1016/j.anai.2016.07.043. PMID 27979015.
- ^ a b Petsky HL, Kew KM, Turner C, Chang AB (September 2016). "Exhaled nitric oxide levels to guide treatment for adults with asthma". The Cochrane Database of Systematic Reviews. 2016 (9): CD011440. doi:10.1002/14651858.CD011440.pub2. PMC 6457753. PMID 27580628.
- ^ a b Petsky HL, Kew KM, Chang AB (November 2016). "Exhaled nitric oxide levels to guide treatment for children with asthma". The Cochrane Database of Systematic Reviews. 11 (5): CD011439. doi:10.1002/14651858.CD011439.pub2. PMC 6432844. PMID 27825189.
- ^ Keeney GE, Gray MP, Morrison AK, Levas MN, Kessler EA, Hill GD, et al. (March 2014). "Dexamethasone for acute asthma exacerbations in children: a meta-analysis". Pediatrics. 133 (3): 493–9. doi:10.1542/peds.2013-2273. PMC 3934336. PMID 24515516.
- ^ Rowe BH, Kirkland SW, Vandermeer B, Campbell S, Newton A, Ducharme FM, Villa-Roel C (March 2017). "Prioritizing Systemic Corticosteroid Treatments to Mitigate Relapse in Adults With Acute Asthma: A Systematic Review and Network Meta-analysis". Academic Emergency Medicine. 24 (3): 371–381. doi:10.1111/acem.13107. PMID 27664401. S2CID 30182169.
- ^ Noppen M (August 2002). "Magnesium treatment for asthma: where do we stand?". Chest. 122 (2): 396–8. doi:10.1378/chest.122.2.396. PMID 12171805.
- ^ Griffiths B, Kew KM (April 2016). "Intravenous magnesium sulfate for treating children with acute asthma in the emergency department" (PDF). The Cochrane Database of Systematic Reviews. 2016 (4): CD011050. doi:10.1002/14651858.CD011050.pub2. PMC 6599814. PMID 27126744.
- ^ Kew KM, Kirtchuk L, Michell CI (May 2014). Kew KM (ed.). "Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department" (PDF). The Cochrane Database of Systematic Reviews. 5 (5): CD010909. doi:10.1002/14651858.CD010909.pub2. PMC 10892514. PMID 24865567.
- ^ a b Knightly R, Milan SJ, Hughes R, Knopp-Sihota JA, Rowe BH, Normansell R, Powell C (November 2017). "Inhaled magnesium sulfate in the treatment of acute asthma". The Cochrane Database of Systematic Reviews. 2017 (11): CD003898. doi:10.1002/14651858.CD003898.pub6. PMC 6485984. PMID 29182799.
- ^ a b Rodrigo GJ, Rodrigo C, Hall JB (March 2004). "Acute asthma in adults: a review". Chest. 125 (3): 1081–102. doi:10.1378/chest.125.3.1081. PMID 15006973.
- ^ GINA 2011, p. 37
- ^ NHLBI Guideline 2007, p. 399
- ^ Jat KR, Chawla D, et al. (Cochrane Airways Group) (November 2012). "Ketamine for management of acute exacerbations of asthma in children". The Cochrane Database of Systematic Reviews. 11 (11): CD009293. doi:10.1002/14651858.CD009293.pub2. PMC 6483733. PMID 23152273.
- ^ a b Castro M, Musani AI, Mayse ML, Shargill NS (April 2010). "Bronchial thermoplasty: a novel technique in the treatment of severe asthma". Therapeutic Advances in Respiratory Disease. 4 (2): 101–16. doi:10.1177/1753465810367505. PMID 20435668.
- ^ Boulet LP, Laviolette M (May–June 2012). "Is there a role for bronchial thermoplasty in the treatment of asthma?". Canadian Respiratory Journal. 19 (3): 191–2. doi:10.1155/2012/853731. PMC 3418092. PMID 22679610.
- ^ GINA 2011, p. 70
- ^ "Pulmonary-Allergy Drugs Advisory Committee Meeting". FDA. July 25, 2018. Retrieved May 9, 2019.
- ^ Sastre J, Dávila I (June 2018). "Dupilumab: A New Paradigm for the Treatment of Allergic Diseases". Journal of Investigational Allergology & Clinical Immunology. 28 (3): 139–150. doi:10.18176/jiaci.0254. hdl:10486/686799. PMID 29939132.
- ^ Israel E, Reddel HK (September 2017). "Severe and Difficult-to-Treat Asthma in Adults". The New England Journal of Medicine. 377 (10): 965–976. doi:10.1056/NEJMra1608969. PMID 28877019. S2CID 44767865.
- ^ McQueen RB, Sheehan DN, Whittington MD, van Boven JF, Campbell JD (August 2018). "Cost-Effectiveness of Biological Asthma Treatments: A Systematic Review and Recommendations for Future Economic Evaluations". PharmacoEconomics. 36 (8): 957–971. doi:10.1007/s40273-018-0658-x. PMID 29736895. S2CID 13681118.
- ^ Farne HA, Wilson A, Milan S, Banchoff E, Yang F, Powell CV (July 2022). "Anti-IL-5 therapies for asthma". The Cochrane Database of Systematic Reviews. 2022 (7): CD010834. doi:10.1002/14651858.CD010834.pub4. PMC 9285134. PMID 35838542.
- ^ Lin SY, Erekosima N, Kim JM, Ramanathan M, Suarez-Cuervo C, Chelladurai Y, et al. (March 2013). "Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review". JAMA. 309 (12): 1278–88. doi:10.1001/jama.2013.2049. PMID 23532243.
- ^ Korang SK, Feinberg J, Wetterslev J, Jakobsen JC (September 2016). "Non-invasive positive pressure ventilation for acute asthma in children". The Cochrane Database of Systematic Reviews. 2016 (9): CD012067. doi:10.1002/14651858.CD012067.pub2. PMC 6457810. PMID 27687114.
- ^ a b c Chan A, De Simoni A, Wileman V, Holliday L, Newby CJ, Chisari C, et al. (Cochrane Airways Group) (June 2022). "Digital interventions to improve adherence to maintenance medication in asthma". The Cochrane Database of Systematic Reviews. 2022 (6): CD013030. doi:10.1002/14651858.CD013030.pub2. PMC 9188849. PMID 35691614.
- ^ Blanc PD, Trupin L, Earnest G, Katz PP, Yelin EH, Eisner MD (November 2001). "Alternative therapies among adults with a reported diagnosis of asthma or rhinosinusitis : data from a population-based survey". Chest. 120 (5): 1461–7. doi:10.1378/chest.120.5.1461. PMID 11713120.
- ^ Shenfield G, Lim E, Allen H (June 2002). "Survey of the use of complementary medicines and therapies in children with asthma". Journal of Paediatrics and Child Health. 38 (3): 252–7. doi:10.1046/j.1440-1754.2002.00770.x. PMID 12047692. S2CID 22129160.
- ^ Milan SJ, Hart A, Wilkinson M (October 2013). "Vitamin C for asthma and exercise-induced bronchoconstriction". The Cochrane Database of Systematic Reviews. 2013 (10): CD010391. doi:10.1002/14651858.CD010391.pub2. PMC 6513466. PMID 24154977.
- ^ Wilkinson M, Hart A, Milan SJ, Sugumar K (June 2014). "Vitamins C and E for asthma and exercise-induced bronchoconstriction". The Cochrane Database of Systematic Reviews. 2014 (6): CD010749. doi:10.1002/14651858.CD010749.pub2. PMC 6513032. PMID 24936673.
- ^ Hemilä H (June 2013). "Vitamin C may alleviate exercise-induced bronchoconstriction: a meta-analysis". BMJ Open. 3 (6): e002416. doi:10.1136/bmjopen-2012-002416. PMC 3686214. PMID 23794586.

- ^ Woods RK, Thien FC, Abramson MJ (2002). "Dietary marine fatty acids (fish oil) for asthma in adults and children". The Cochrane Database of Systematic Reviews. 2019 (3): CD001283. doi:10.1002/14651858.CD001283. PMC 6436486. PMID 12137622.
- ^ Pogson Z, McKeever T (March 2011). "Dietary sodium manipulation and asthma". The Cochrane Database of Systematic Reviews. 2011 (3): CD000436. doi:10.1002/14651858.CD000436.pub3. PMC 7032646. PMID 21412865.
- ^ a b Williamson A, Martineau AR, Sheikh A, Jolliffe D, Griffiths CJ (February 2023). "Vitamin D for the management of asthma". The Cochrane Database of Systematic Reviews. 2023 (2): CD011511. doi:10.1002/14651858.CD011511.pub3. PMC 9899558. PMID 36744416.
- ^ a b Zhou Y, Yang M, Dong BR (June 2012). "Monosodium glutamate avoidance for chronic asthma in adults and children". The Cochrane Database of Systematic Reviews. 2014 (6): CD004357. doi:10.1002/14651858.CD004357.pub4. PMC 8823518. PMID 22696342.
- ^ a b NHLBI Guideline 2007, p. 240
- ^ McCarney RW, Brinkhaus B, Lasserson TJ, Linde K (2004). McCarney RW (ed.). "Acupuncture for chronic asthma". The Cochrane Database of Systematic Reviews. 2009 (1): CD000008. doi:10.1002/14651858.CD000008.pub2. PMC 7061358. PMID 14973944.
- ^ Blackhall K, Appleton S, Cates CJ (September 2012). Blackhall K (ed.). "Ionisers for chronic asthma". The Cochrane Database of Systematic Reviews. 2017 (9): CD002986. doi:10.1002/14651858.CD002986.pub2. PMC 6483773. PMID 22972060.
- ^ Hondras MA, Linde K, Jones AP (April 2005). Hondras MA (ed.). "Manual therapy for asthma". The Cochrane Database of Systematic Reviews (2): CD001002. doi:10.1002/14651858.CD001002.pub2. PMID 15846609.
- ^ Osadnik CR, Gleeson C, McDonald VM, Holland AE, et al. (Cochrane Airways Group) (August 2022). "Pulmonary rehabilitation versus usual care for adults with asthma". The Cochrane Database of Systematic Reviews. 2022 (8): CD013485. doi:10.1002/14651858.CD013485.pub2. PMC 9394585. PMID 35993916.
- ^ Macêdo TM, Freitas DA, Chaves GS, Holloway EA, Mendonça KM (April 2016). "Breathing exercises for children with asthma". The Cochrane Database of Systematic Reviews. 2016 (4): CD011017. doi:10.1002/14651858.CD011017.pub2. PMC 7104663. PMID 27070225.
- ^ Sergel MJ, Cydulka RK (September 2009). "Ch. 75: Asthma". In Wolfson AB, Harwood-Nuss A (eds.). Harwood-Nuss' Clinical Practice of Emergency Medicine (5th ed.). Lippincott Williams & Wilkins. pp. 432–. ISBN 978-0-7817-8943-1.
- ^ NHLBI Guideline 2007, p. 1
- ^ a b "The Global Asthma Report 2014". Archived from the original on April 27, 2016. Retrieved May 10, 2016.
- ^ The global burden of disease : 2004 update ([Online-Ausg.] ed.). Geneva: World Health Organization. 2008. p. 35. ISBN 978-92-4-156371-0.
- ^ Maddox L, Schwartz DA (2002). "The pathophysiology of asthma". Annual Review of Medicine. 53: 477–98. doi:10.1146/annurev.med.53.082901.103921. PMID 11818486.
- ^ Hahn DL (2022). "Does the asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS) exist? A narrative review from epidemiology and practice". Allergol Immunopathol (Madr). 50 (6): 100–106. doi:10.15586/aei.v50i6.678. PMID 36335452.
- ^ Beckett PA, Howarth PH (February 2003). "Pharmacotherapy and airway remodelling in asthma?". Thorax. 58 (2): 163–74. doi:10.1136/thorax.58.2.163. PMC 1746582. PMID 12554904.
- ^ Silva N, Carona C, Crespo C, Canavarro MC (June 2015). "Quality of life in pediatric asthma patients and their parents: a meta-analysis on 20 years of research". Expert Review of Pharmacoeconomics & Outcomes Research. 15 (3): 499–519. doi:10.1586/14737167.2015.1008459. hdl:10316/45410. PMID 25651982. S2CID 8768325.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on November 11, 2009. Retrieved November 11, 2009.
- ^ "Asthma prevalence". Our World in Data. Retrieved February 15, 2020.
- ^ World Health Organization. "WHO: Asthma". Archived from the original on December 15, 2007. Retrieved December 29, 2007.
- ^ Bush A, Menzies-Gow A (December 2009). "Phenotypic differences between pediatric and adult asthma". Proceedings of the American Thoracic Society. 6 (8): 712–719. doi:10.1513/pats.200906-046DP. PMID 20008882.
- ^ Weiss AJ, Wier LM, Stocks C, Blanchard J (June 2014). "Overview of Emergency Department Visits in the United States, 2011". HCUP Statistical Brief (174). Agency for Healthcare Research and Quality. PMID 25144109. Archived from the original on August 3, 2014.
- ^ Martin MA, Press VG, Nyenhuis SM, Krishnan JA, Erwin K, Mosnaim G, et al. (December 2016). "Care transition interventions for children with asthma in the emergency department". The Journal of Allergy and Clinical Immunology. 138 (6): 1518–1525. doi:10.1016/j.jaci.2016.10.012. PMC 5327498. PMID 27931533.
- ^ Grant EN, Wagner R, Weiss KB (August 1999). "Observations on emerging patterns of asthma in our society". The Journal of Allergy and Clinical Immunology. 104 (2 Pt 2): S1-9. doi:10.1016/S0091-6749(99)70268-X. PMID 10452783.
- ^ Bousquet J, Bousquet PJ, Godard P, Daures JP (July 2005). "The public health implications of asthma". Bulletin of the World Health Organization. 83 (7): 548–54. PMC 2626301. PMID 16175830.
- ^ Anderson HR, Gupta R, Strachan DP, Limb ES (January 2007). "50 years of asthma: UK trends from 1955 to 2004". Thorax. 62 (1): 85–90. doi:10.1136/thx.2006.066407. PMC 2111282. PMID 17189533.
- ^ Masoli M (2004). Global Burden of Asthma (PDF). p. 9. Archived from the original (PDF) on May 2, 2013.
- ^ "Asthma-related death rate in UK among highest in Europe, charity analysis finds". Pharmaceutical Journal. May 3, 2018. Archived from the original on July 26, 2020. Retrieved August 13, 2018.
- ^ "Asthma attacks triple when children return to school in September". NHS UK. July 3, 2019. Archived from the original on July 26, 2020. Retrieved August 23, 2019.
- ^ Rantala A, Jaakkola JJ, Jaakkola MS (2011). "Respiratory infections precede adult-onset asthma". PLOS ONE. 6 (12): e27912. Bibcode:2011PLoSO...627912R. doi:10.1371/journal.pone.0027912. PMC 3244385. PMID 22205932.
- ^ Yeh JJ, Wang YC, Hsu WH, Kao CH (April 2016). "Incident asthma and Mycoplasma pneumoniae: A nationwide cohort study". The Journal of Allergy and Clinical Immunology. 137 (4): 1017–1023.e6. doi:10.1016/j.jaci.2015.09.032. PMID 26586037.
- ^ a b c Barrett ML, Wier LM, Washington R (January 2014). "Trends in Pediatric and Adult Hospital Stays for Asthma, 2000–2010". HCUP Statistical Brief (169). Rockville, MD: Agency for Healthcare Research and Quality. PMID 24624462. Archived from the original on March 28, 2014.
- ^ Rosner F (2002). "The Life of Moses Maimonides, a Prominent Medieval Physician" (PDF). Einstein Quart J Biol Med. 19 (3): 125–28. Archived (PDF) from the original on March 5, 2009.
- ^ C14 Chinese medication chart; Asthma etc. Wellcome L0039608
- ^ Thorowgood JC (November 1873). "On Bronchial Asthma". British Medical Journal. 2 (673): 600. doi:10.1136/bmj.2.673.600. PMC 2294647. PMID 20747287.
- ^ Gaskoin G (March 1872). "On the Treatment of Asthma". British Medical Journal. 1 (587): 339. doi:10.1136/bmj.1.587.339. PMC 2297349. PMID 20746575.
- ^ Berkart JB (June 1880). "The Treatment of Asthma". British Medical Journal. 1 (1016): 917–8. doi:10.1136/bmj.1.1016.917. PMC 2240555. PMID 20749537.
Berkart JB (June 1880). "The Treatment of Asthma". British Medical Journal. 1 (1017): 960–2. doi:10.1136/bmj.1.1017.960. PMC 2240530. PMID 20749546. - ^ Bosworth FH (1886). "Hay Fever, Asthma, and Allied Affections". Transactions of the Annual Meeting of the American Climatological Association. 2: 151–70. PMC 2526599. PMID 21407325.
- ^ Sanders MJ (2017). "Guiding Inspiratory Flow: Development of the In-Check DIAL G16, a Tool for Improving Inhaler Technique". Pulmonary Medicine. 2017: 1–7. doi:10.1155/2017/1495867. ISSN 2090-1836. PMC 5733915. PMID 29348936.
- ^ Kapri A, Pant S, Gupta N, Paliwal S, Nain S (November 11, 2022). "Asthma History, Current Situation, an Overview of Its Control History, Challenges, and Ongoing Management Programs: An Updated Review". Proceedings of the National Academy of Sciences, India Section B. 93 (3): 539–551. doi:10.1007/s40011-022-01428-1. ISSN 0369-8211. PMC 9651109. PMID 36406816.
- ^ Doig RL (February 1905). "Epinephrin; Especially in Asthma". California State Journal of Medicine. 3 (2): 54–5. PMC 1650334. PMID 18733372.
- ^ von Mutius E, Drazen JM (March 2012). "A patient with asthma seeks medical advice in 1828, 1928, and 2012". The New England Journal of Medicine. 366 (9): 827–34. doi:10.1056/NEJMra1102783. PMID 22375974. S2CID 5143546.
- ^ Crompton G (December 2006). "A brief history of inhaled asthma therapy over the last fifty years". Primary Care Respiratory Journal. 15 (6): 326–331. doi:10.1016/j.pcrj.2006.09.002. PMC 6730840. PMID 17092772.
- ^ McCullough D (1981). Mornings on Horseback: The Story of an Extraordinary Family, a Vanished Way of Life and the Unique Child Who Became Theodore Roosevelt. Simon and Schuster. pp. 93–108. ISBN 978-0-7432-1830-6. Archived from the original on April 7, 2015.
- ^ a b Opolski M, Wilson I (September 2005). "Asthma and depression: a pragmatic review of the literature and recommendations for future research". Clinical Practice and Epidemiology in Mental Health. 1: 18. doi:10.1186/1745-0179-1-18. PMC 1253523. PMID 16185365.
- ^ "Bangladeshi man with asthma wins France deportation fight". The Guardian. January 12, 2021. Retrieved January 12, 2021.
References
- National Asthma Education and Prevention Program (2007). "Guidelines for the Diagnosis and Management of Asthma". National Heart Lung and Blood Institute. EPR-3.
- "British Guideline on the Management of Asthma" (PDF). British Thoracic Society. 2012 [2008]. SIGN 101. Archived from the original (PDF) on August 19, 2008. Retrieved August 4, 2008.
- British Guideline on the Management of Asthma. British Thoracic Society. July 2019. ISBN 978-1-909103-70-2. SIGN 158.
- "Global Strategy for Asthma Management and Prevention" (PDF). Global Initiative for Asthma. 2011. Archived Reports.
External links